Ultrastable metallic glasses formed on cold substrates
- PMID: 29643346
- PMCID: PMC5895802
- DOI: 10.1038/s41467-018-03656-4
Ultrastable metallic glasses formed on cold substrates
Abstract
Vitrification from physical vapor deposition is known to be an efficient way for tuning the kinetic and thermodynamic stability of glasses and significantly improve their properties. There is a general consensus that preparing stable glasses requires the use of high substrate temperatures close to the glass transition one, Tg. Here, we challenge this empirical rule by showing the formation of Zr-based ultrastable metallic glasses (MGs) at room temperature, i.e., with a substrate temperature of only 0.43Tg. By carefully controlling the deposition rate, we can improve the stability of the obtained glasses to higher values. In contrast to conventional quenched glasses, the ultrastable MGs exhibit a large increase of Tg of ∼60 K, stronger resistance against crystallization, and more homogeneous structure with less order at longer distances. Our study circumvents the limitation of substrate temperature for developing ultrastable glasses, and provides deeper insight into glasses stability and their surface dynamics.
Conflict of interest statement
The authors declare no competing interests.
Figures


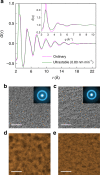
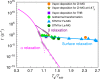
References
-
- Dyre JC. Colloquium: the glass transition and elastic models of glass-forming liquids. Rev. Mod. Phys. 2006;78:953–972. doi: 10.1103/RevModPhys.78.953. - DOI
-
- Badrinarayanan P, Zheng W, Li Q, Simon SL. The glass transition temperature versus the fictive temperature. J. Non Cryst. Solids. 2007;353:2603–2612. doi: 10.1016/j.jnoncrysol.2007.04.025. - DOI
-
- Liu ZY, et al. Cooling rate effect on Young’s modulus and hardness of a Zr-based metallic glass. J. Alloys Compd. 2011;509:3269–3273. doi: 10.1016/j.jallcom.2010.12.095. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

