Catalytically active inclusion bodies of L-lysine decarboxylase from E. coli for 1,5-diaminopentane production
- PMID: 29643457
- PMCID: PMC5895699
- DOI: 10.1038/s41598-018-24070-2
Catalytically active inclusion bodies of L-lysine decarboxylase from E. coli for 1,5-diaminopentane production
Abstract
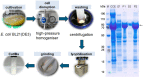
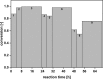
Sustainable and eco-efficient alternatives for the production of platform chemicals, fuels and chemical building blocks require the development of stable, reusable and recyclable biocatalysts. Here we present a novel concept for the biocatalytic production of 1,5-diaminopentane (DAP, trivial name: cadaverine) using catalytically active inclusion bodies (CatIBs) of the constitutive L-lysine decarboxylase from E. coli (EcLDCc-CatIBs) to process L-lysine-containing culture supernatants from Corynebacterium glutamicum. EcLDCc-CatIBs can easily be produced in E. coli followed by a simple purification protocol yielding up to 43% dry CatIBs per dry cell weight. The stability and recyclability of EcLDCc-CatIBs was demonstrated in (repetitive) batch experiments starting from L-lysine concentrations of 0.1 M and 1 M. EcLDC-CatIBs exhibited great stability under reaction conditions with an estimated half-life of about 54 h. High conversions to DAP of 87-100% were obtained in 30-60 ml batch reactions using approx. 180-300 mg EcLDCc-CatIBs, respectively. This resulted in DAP titres of up to 88.4 g l-1 and space-time yields of up to 660 gDAP l-1 d-1 per gram dry EcLDCc-CatIBs. The new process for DAP production can therefore compete with the currently best fermentative process as described in the literature.
Conflict of interest statement
The authors declare no competing interests.
Figures
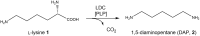




References
-
- Kriegler E, et al. Fossil-fueled development (SSP5): An energy and resource intensive scenario for the 21st century. Glob. Environ. Chang. 2017;42:297–315. doi: 10.1016/j.gloenvcha.2016.05.015. - DOI
-
- U.S. Energy Information Administration. International Energy Outlook 2016. 0484, (2016).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

