Functional circuit architecture underlying parental behaviour
- PMID: 29643503
- PMCID: PMC5908752
- DOI: 10.1038/s41586-018-0027-0
Functional circuit architecture underlying parental behaviour
Abstract
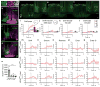
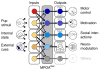
Parenting is essential for the survival and wellbeing of mammalian offspring. However, we lack a circuit-level understanding of how distinct components of this behaviour are coordinated. Here we investigate how galanin-expressing neurons in the medial preoptic area (MPOAGal) of the hypothalamus coordinate motor, motivational, hormonal and social aspects of parenting in mice. These neurons integrate inputs from a large number of brain areas and the activation of these inputs depends on the animal's sex and reproductive state. Subsets of MPOAGal neurons form discrete pools that are defined by their projection sites. While the MPOAGal population is active during all episodes of parental behaviour, individual pools are tuned to characteristic aspects of parenting. Optogenetic manipulation of MPOAGal projections mirrors this specificity, affecting discrete parenting components. This functional organization, reminiscent of the control of motor sequences by pools of spinal cord neurons, provides a new model for how discrete elements of a social behaviour are generated at the circuit level.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





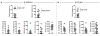





Comment in
-
Circuit Architecture Underlying Distinct Components of Parental Care.Trends Neurosci. 2018 Jun;41(6):334-336. doi: 10.1016/j.tins.2018.04.003. Epub 2018 Apr 21. Trends Neurosci. 2018. PMID: 29685403
-
Social Behavior: How the Brain Thinks like a Mom.Curr Biol. 2018 Jul 9;28(13):R746-R749. doi: 10.1016/j.cub.2018.05.049. Curr Biol. 2018. PMID: 29990458
References
-
- Numan M, Insel TR. The neurobiology of parental behavior. Springer; 2011.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

