Aspm knockout ferret reveals an evolutionary mechanism governing cerebral cortical size
- PMID: 29643508
- PMCID: PMC6095461
- DOI: 10.1038/s41586-018-0035-0
Aspm knockout ferret reveals an evolutionary mechanism governing cerebral cortical size
Abstract
The human cerebral cortex is distinguished by its large size and abundant gyrification, or folding. However, the evolutionary mechanisms that drive cortical size and structure are unknown. Although genes that are essential for cortical developmental expansion have been identified from the genetics of human primary microcephaly (a disorder associated with reduced brain size and intellectual disability) 1 , studies of these genes in mice, which have a smooth cortex that is one thousand times smaller than the cortex of humans, have provided limited insight. Mutations in abnormal spindle-like microcephaly-associated (ASPM), the most common recessive microcephaly gene, reduce cortical volume by at least 50% in humans2-4, but have little effect on the brains of mice5-9; this probably reflects evolutionarily divergent functions of ASPM10,11. Here we used genome editing to create a germline knockout of Aspm in the ferret (Mustela putorius furo), a species with a larger, gyrified cortex and greater neural progenitor cell diversity12-14 than mice, and closer protein sequence homology to the human ASPM protein. Aspm knockout ferrets exhibit severe microcephaly (25-40% decreases in brain weight), reflecting reduced cortical surface area without significant change in cortical thickness, as has been found in human patients3,4, suggesting that loss of 'cortical units' has occurred. The cortex of fetal Aspm knockout ferrets displays a very large premature displacement of ventricular radial glial cells to the outer subventricular zone, where many resemble outer radial glia, a subtype of neural progenitor cells that are essentially absent in mice and have been implicated in cerebral cortical expansion in primates12-16. These data suggest an evolutionary mechanism by which ASPM regulates cortical expansion by controlling the affinity of ventricular radial glial cells for the ventricular surface, thus modulating the ratio of ventricular radial glial cells, the most undifferentiated cell type, to outer radial glia, a more differentiated progenitor.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

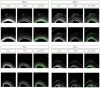
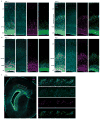



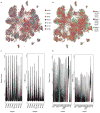





Comment in
-
An expanding role.Nat Rev Neurosci. 2018 Jun;19(6):320-321. doi: 10.1038/s41583-018-0017-0. Nat Rev Neurosci. 2018. PMID: 29740173 No abstract available.
References
-
- Passemard S, et al. Abnormal spindle-like microcephaly-associated (ASPM) mutations strongly disrupt neocortical structure but spare the hippocampus and long-term memory. Cortex; a journal devoted to the study of the nervous system and behavior. 2016;74:158–176. doi: 10.1016/j.cortex.2015.10.010. - DOI - PubMed
-
- Pulvers JN, et al. Mutations in mouse Aspm (abnormal spindle-like microcephaly associated) cause not only microcephaly but also major defects in the germline. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16595–16600. doi: 10.1073/pnas.1010494107. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 ES005605/ES/NIEHS NIH HHS/United States
- P30 NS052519/NS/NINDS NIH HHS/United States
- F32 NS100338/NS/NINDS NIH HHS/United States
- R01 MH067528/MH/NIMH NIH HHS/United States
- R01 NS035129/NS/NINDS NIH HHS/United States
- R21 NS091865/NS/NINDS NIH HHS/United States
- K99 NS112604/NS/NINDS NIH HHS/United States
- R24 HL123482/HL/NHLBI NIH HHS/United States
- R21 HD083956/HD/NICHD NIH HHS/United States
- R01 NS032457/NS/NINDS NIH HHS/United States
- R24 MH114805/MH/NIMH NIH HHS/United States
- R01 EB017337/EB/NIBIB NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

