Tanezumab: Therapy targeting nerve growth factor in pain pathogenesis
- PMID: 29643634
- PMCID: PMC5885425
- DOI: 10.4103/joacp.JOACP_389_15
Tanezumab: Therapy targeting nerve growth factor in pain pathogenesis
Abstract
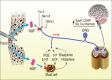
In recent years, nerve growth factor (NGF) and the NGF receptor have become potential therapeutic targets in the treatment of acute and chronic pain states. NGF is a neurotrophin involved in regulating the function of sensory and sympathetic neurons during development. Numerous pain states have been linked to elevated levels of NGF and its role in increasing the perception of pain. Tanezumab, a recombinant humanized monoclonal antibody (IgG), was developed to target NGF, binding both circulating and local tissue NGF preventing interaction with the tropomyosin-related kinase-A and p75 receptors. Recent clinical studies with tanezumab in different patient populations to date, including osteoarthritis, low back pain, and diabetic peripheral neuropathy, demonstrate efficacy with few side effects, including transient arthralgias, paresthesias, hypoesthesia, and rarely, osteonecrosis. Anti-NGF antibodies are a novel therapy in pain management and have shown promise in the treatment of certain pain conditions, which at present are poorly treated. Tanezumab offers an exciting new class of analgesics that has the potential to change the treatment of pain.
Keywords: Monoclonal antibody; nerve growth factor; neuropathic pain; tanezumab; tropomyosin-related kinase.
Conflict of interest statement
There are no conflicts of interest.
Figures
Comment in
-
Response to: "Tanezumab: Therapy targeting nerve growth factor in pain pathogenesis".J Anaesthesiol Clin Pharmacol. 2018 Oct-Dec;34(4):548. doi: 10.4103/joacp.JOACP_155_18. J Anaesthesiol Clin Pharmacol. 2018. PMID: 30774242 Free PMC article. No abstract available.
References
-
- Institute of Medicine, the National Institutes of Health. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education and Research. [Last accessed on 2015 Dec 12]. Available from: https://www.iom.nationalacademies.org/~/media/Files/Report%20Files/2011/... .
-
- Bhangare KP, Kaye AD, Knezevic NN, Candido KD, Urman RD. An Analysis of New Approaches and Drug Formulations for Treatment of Chronic Low Back Pain. Anesthesiol Clin. 2017;;35:341–50. - PubMed
-
- Vadivelu N, Gowda AM, Urman RD, Jolly S, Kodumudi V, Maria M, et al. Ketorolac tromethamine-routes and clinical implications. Pain Pract. 2015;15:175–93. - PubMed
-
- Chou R, Huffman LH. Medications for acute and chronic low back pain: A review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492–504. - PubMed
-
- Food and Drug Administration Center for Drug Evaluation and Research Background Materials. Meeting of the Arthritis Advisory Committee (AAC) [Last accessed on 2016 Mar 10]. Available from: http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmateria... .
LinkOut - more resources
Full Text Sources
Research Materials