Antimicrobial Peptides and Their Therapeutic Potential for Bacterial Skin Infections and Wounds
- PMID: 29643807
- PMCID: PMC5882822
- DOI: 10.3389/fphar.2018.00281
Antimicrobial Peptides and Their Therapeutic Potential for Bacterial Skin Infections and Wounds
Abstract
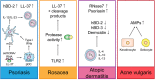
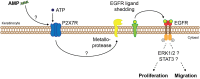
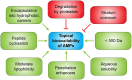
Alarming data about increasing resistance to conventional antibiotics are reported, while at the same time the development of new antibiotics is stagnating. Skin and soft tissue infections (SSTIs) are mainly caused by the so called ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) which belong to the most recalcitrant bacteria and are resistant to almost all common antibiotics. S. aureus and P. aeruginosa are the most frequent pathogens isolated from chronic wounds and increasing resistance to topical antibiotics has become a major issue. Therefore, new treatment options are urgently needed. In recent years, research focused on the development of synthetic antimicrobial peptides (AMPs) with lower toxicity and improved activity compared to their endogenous counterparts. AMPs appear to be promising therapeutic options for the treatment of SSTIs and wounds as they show a broad spectrum of antimicrobial activity, low resistance rates and display pivotal immunomodulatory as well as wound healing promoting activities such as induction of cell migration and proliferation and angiogenesis. In this review, we evaluate the potential of AMPs for the treatment of bacterial SSTIs and wounds and provide an overview of the mechanisms of actions of AMPs that contribute to combat skin infections and to improve wound healing. Bacteria growing in biofilms are more resistant to conventional antibiotics than their planktonic counterparts due to limited biofilm penetration and distinct metabolic and physiological functions, and often result in chronification of infections and wounds. Thus, we further discuss the feasibility of AMPs as anti-biofilm agents. Finally, we highlight perspectives for future therapies and which issues remain to bring AMPs successfully to the market.
Keywords: antimicrobial peptides; bacterial resistance; bacterial toxins; biofilms; skin and soft tissue infections; topical therapy; wound healing; wounds.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

