Loss of neutrophil polarization in colon carcinoma liver metastases of mice with an inducible, liver-specific IGF-I deficiency
- PMID: 29644002
- PMCID: PMC5884657
- DOI: 10.18632/oncotarget.24593
Loss of neutrophil polarization in colon carcinoma liver metastases of mice with an inducible, liver-specific IGF-I deficiency
Abstract
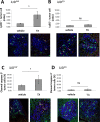
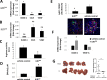
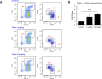
The growth of cancer metastases in the liver depends on a permissive interaction with the hepatic microenvironment and neutrophils can contribute to this interaction, either positively or negatively, depending on their phenotype. Here we investigated the role of IGF-I in the control of the tumor microenvironment in the liver, using mice with a conditional, liver-specific, IGF-I deficiency (iLID) induced by a single tamoxifen injection. In mice that had a sustained (3 weeks) IGF-I deficiency prior to the intrasplenic/portal inoculation of colon carcinoma MC-38 cells, we observed an increase in neutrophil accumulation in the liver relative to controls. However, unlike controls, these neutrophils did not acquire the (anti-inflammatory) tumor-promoting phenotype, as evidenced by retention of high ICAM-1 expression and nitric oxide production and low CXCR4, CCL5, and VEGF expression and arginase production, all characteristic of the (pro-inflammatory) phenotype. This coincided with an increase in apoptotic tumor cells and reduced metastasis. Neutrophils isolated from these mice also had reduced IGF-IR expression levels. These changes were not observed in iLID mice with a short-term (2 days) IGF-I depletion, despite a 70% reduction in their circulating IGF-I levels, indicating that a sustained IGF-I deficiency was necessary to alter the neutrophil phenotype. Similar results were obtained with the highly metastatic Lewis lung carcinoma subline H-59 cells and in mice injected with an IGF-Trap that blocks IGF-IR signaling by reducing ligand bioavailability. Our results implicate the IGF axis in neutrophil polarization and the induction of a pro-metastatic microenvironment in the liver.
Keywords: IGF-I; colorectal carcinoma; liver metastasis; neutrophil polarization; tumor microenvironment.
Conflict of interest statement
CONFLICTS OF INTEREST The authors have no conflict of interest to declare.
Figures



Similar articles
-
Insulin-like growth factor-I regulates the liver microenvironment in obese mice and promotes liver metastasis.Cancer Res. 2010 Jan 1;70(1):57-67. doi: 10.1158/0008-5472.CAN-09-2472. Cancer Res. 2010. PMID: 20048072 Free PMC article.
-
The type I insulin-like growth factor regulates the liver stromal response to metastatic colon carcinoma cells.Oncotarget. 2016 Oct 12;8(32):52281-52293. doi: 10.18632/oncotarget.12595. eCollection 2017 Aug 8. Oncotarget. 2016. PMID: 28881729 Free PMC article.
-
HRG inhibits liver cancer lung metastasis by suppressing neutrophil extracellular trap formation.Clin Transl Med. 2023 Jun;13(6):e1283. doi: 10.1002/ctm2.1283. Clin Transl Med. 2023. PMID: 37254661 Free PMC article.
-
Leveling Up the Controversial Role of Neutrophils in Cancer: When the Complexity Becomes Entangled.Cells. 2021 Sep 20;10(9):2486. doi: 10.3390/cells10092486. Cells. 2021. PMID: 34572138 Free PMC article. Review.
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
Cited by
-
The role of liver microenvironment in hepatic metastasis.Clin Transl Med. 2019 Jul 1;8(1):21. doi: 10.1186/s40169-019-0237-6. Clin Transl Med. 2019. PMID: 31263976 Free PMC article. Review.
-
Liver metastases.Nat Rev Dis Primers. 2021 Apr 15;7(1):27. doi: 10.1038/s41572-021-00261-6. Nat Rev Dis Primers. 2021. PMID: 33859205 Review.
-
Targeting the IGF-Axis Potentiates Immunotherapy for Pancreatic Ductal Adenocarcinoma Liver Metastases by Altering the Immunosuppressive Microenvironment.Mol Cancer Ther. 2021 Dec;20(12):2469-2482. doi: 10.1158/1535-7163.MCT-20-0144. Epub 2021 Sep 22. Mol Cancer Ther. 2021. PMID: 34552012 Free PMC article.
-
Molecular Mechanisms of Colorectal Liver Metastases.Cells. 2023 Jun 18;12(12):1657. doi: 10.3390/cells12121657. Cells. 2023. PMID: 37371127 Free PMC article. Review.
-
Targeting the IGF-Axis for Cancer Therapy: Development and Validation of an IGF-Trap as a Potential Drug.Cells. 2020 Apr 29;9(5):1098. doi: 10.3390/cells9051098. Cells. 2020. PMID: 32365498 Free PMC article. Review.
References
-
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. - PubMed
-
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

