The long non-coding RNA HOTAIR is transcriptionally activated by HOXA9 and is an independent prognostic marker in patients with malignant glioma
- PMID: 29644006
- PMCID: PMC5884661
- DOI: 10.18632/oncotarget.24597
The long non-coding RNA HOTAIR is transcriptionally activated by HOXA9 and is an independent prognostic marker in patients with malignant glioma
Abstract
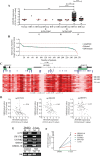
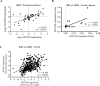
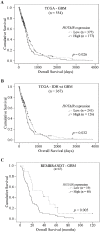
The lncRNA HOTAIR has been implicated in several human cancers. Here, we evaluated the molecular alterations and upstream regulatory mechanisms of HOTAIR in glioma, the most common primary brain tumors, and its clinical relevance. HOTAIR gene expression, methylation, copy-number and prognostic value were investigated in human gliomas integrating data from online datasets and our cohorts. High levels of HOTAIR were associated with higher grades of glioma, particularly IDH wild-type cases. Mechanistically, HOTAIR was overexpressed in a gene dosage-independent manner, while DNA methylation levels of particular CpGs in HOTAIR locus were associated with HOTAIR expression levels in GBM clinical specimens and cell lines. Concordantly, the demethylating agent 5-Aza-2'-deoxycytidine affected HOTAIR transcriptional levels in a cell line-dependent manner. Importantly, HOTAIR was frequently co-expressed with HOXA9 in high-grade gliomas from TCGA, Oncomine, and our Portuguese and French datasets. Integrated in silico analyses, chromatin immunoprecipitation, and qPCR data showed that HOXA9 binds directly to the promoter of HOTAIR. Clinically, GBM patients with high HOTAIR expression had a significantly reduced overall survival, independently of other prognostic variables. In summary, this work reveals HOXA9 as a novel direct regulator of HOTAIR, and establishes HOTAIR as an independent prognostic marker, providing new therapeutic opportunities to treat this highly aggressive cancer.
Keywords: HOTAIR; HOXA9; glioblastoma; glioma; prognosis.
Conflict of interest statement
CONFLICTS OF INTEREST The authors disclose no potential conflicts of interest.
Figures

Similar articles
-
A novel molecular link between HOXA9 and WNT6 in glioblastoma identifies a subgroup of patients with particular poor prognosis.Mol Oncol. 2020 Jun;14(6):1224-1241. doi: 10.1002/1878-0261.12633. Epub 2020 May 6. Mol Oncol. 2020. PMID: 31923345 Free PMC article.
-
Serum long noncoding RNA HOTAIR as a novel diagnostic and prognostic biomarker in glioblastoma multiforme.Mol Cancer. 2018 Mar 20;17(1):74. doi: 10.1186/s12943-018-0822-0. Mol Cancer. 2018. PMID: 29558959 Free PMC article.
-
Effects of the functional HOTAIR rs920778 and rs12826786 genetic variants in glioma susceptibility and patient prognosis.J Neurooncol. 2017 Mar;132(1):27-34. doi: 10.1007/s11060-016-2345-0. Epub 2017 Jan 12. J Neurooncol. 2017. PMID: 28083786
-
HOTAIR is a therapeutic target in glioblastoma.Oncotarget. 2015 Apr 10;6(10):8353-65. doi: 10.18632/oncotarget.3229. Oncotarget. 2015. PMID: 25823657 Free PMC article.
-
HOTAIR in cancer: diagnostic, prognostic, and therapeutic perspectives.Cancer Cell Int. 2024 Dec 19;24(1):415. doi: 10.1186/s12935-024-03612-x. Cancer Cell Int. 2024. PMID: 39702144 Free PMC article. Review.
Cited by
-
Role of HOXA9 in solid tumors: mechanistic insights and therapeutic potential.Cancer Cell Int. 2022 Nov 14;22(1):349. doi: 10.1186/s12935-022-02767-9. Cancer Cell Int. 2022. PMID: 36376832 Free PMC article. Review.
-
Classifying gastric cancer using FLORA reveals clinically relevant molecular subtypes and highlights LINC01614 as a biomarker for patient prognosis.Oncogene. 2021 Apr;40(16):2898-2909. doi: 10.1038/s41388-021-01743-3. Epub 2021 Mar 19. Oncogene. 2021. PMID: 33742127 Free PMC article.
-
A HOTAIR-associated super-enhancer orchestrates glioblastoma malignancy via MEST.Oncogenesis. 2025 Apr 7;14(1):8. doi: 10.1038/s41389-025-00551-8. Oncogenesis. 2025. PMID: 40195296 Free PMC article.
-
Coding of Glioblastoma Progression and Therapy Resistance through Long Noncoding RNAs.Cancers (Basel). 2020 Jul 8;12(7):1842. doi: 10.3390/cancers12071842. Cancers (Basel). 2020. PMID: 32650527 Free PMC article. Review.
-
Exploring the roles and clinical potential of exosome-derived non-coding RNAs in glioma.IBRO Neurosci Rep. 2025 Feb 5;18:323-337. doi: 10.1016/j.ibneur.2025.01.015. eCollection 2025 Jun. IBRO Neurosci Rep. 2025. PMID: 40034544 Free PMC article. Review.
References
-
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. World Health Organization Histological Classification of Tumours of the Central Nervous System. France: International Agency for Research on Cancer; 2016.
-
- Preusser M, de Ribaupierre S, Wohrer A, Erridge SC, Hegi M, Weller M, Stupp R. Current concepts and management of glioblastoma. Ann Neurol. 2011;70:9–21. https://doi.org/10.1002/ana.22425. - DOI - PubMed
-
- Bai RY, Staedtke V, Riggins GJ. Molecular targeting of glioblastoma: Drug discovery and therapies. Trends Mol Med. 2011;17:301–12. https://doi.org/S1471-4914(11)00012-810.1016/j.molmed.2011.01.011. - PMC - PubMed
-
- Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2011;59:1169–80. https://doi.org/10.1002/glia.21136. - DOI - PubMed
-
- Hadziahmetovic M, Shirai K, Chakravarti A. Recent advancements in multimodality treatment of gliomas. Future Oncol. 2011;7:1169–83. https://doi.org/10.2217/fon.11.102. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

