Morphine-alcohol treatment impairs cognitive functions and increases neuro-inflammatory responses in the medial prefrontal cortex of juvenile male rats
- PMID: 29644109
- PMCID: PMC5890016
- DOI: 10.5115/acb.2018.51.1.41
Morphine-alcohol treatment impairs cognitive functions and increases neuro-inflammatory responses in the medial prefrontal cortex of juvenile male rats
Abstract
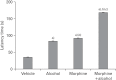
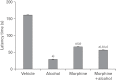
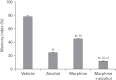
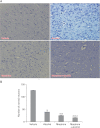
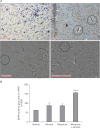
In the developed and developing world, opioid consumption in combination with alcohol has become one of the substances abused. In this experiment, we examined the effects of alcohol, morphine, and morphine+alcohol combination on cognitive functions and neuroinflammatory responses in the medial prefrontal cortex (mPFC) of juvenile male rats. Alcohol (1.0 ml of 15% v/v ethanol twice daily, subcutaneously, 7 hours apart), morphine (0.5 ml/kg of 0.4 mg/kg morphine chlorate twice daily, subcutaneously, 7 hours apart), morphine+alcohol co-treatment (0.5 ml/kg of 0.4 mg/kg morphine chlorate+1.0 ml of 15% v/v ethanol twice daily, subcutaneously, 7 hours apart) were administered for 21 days. Treatment with morphine+alcohol significantly impairs cognition functions in the Morris water maze, passive avoidance, and novel object recognition tests, furthermore, the treatment significantly increased the quantitative count of astrocytic cells and also conferred marked neuronal cell death in the mPFC, which were studied by glial fibrillary acidic protein immunochemistry for astrocytes and Cresyl violet for Nissl's substance distribution in neurons respectively. These results suggest that alcohol, morphine, and morphine+alcohol co-treatment may trigger cognitive deficits and neuroinflammatory responses in the brain.
Keywords: Alcohols; Astrocytes; Cognition; Morphine; Neurodegeneration.
Figures

References
-
- Kopnisky KL, Hyman SE. Molecular and cellular biology of addiction. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Brentwood, TN: merican College of Neuropsychopharmacology; 2002. pp. 1367–1379.
-
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. - PubMed
-
- Martini L, Whistler JL. The role of mu opioid receptor desensitization and endocytosis in morphine tolerance and dependence. Curr Opin Neurobiol. 2007;17:556–564. - PubMed
-
- Dumbili E. Changing patterns of alcohol consumption in Nigeria: an exploration of responsible factors and consequences. Med Soc Online. 2013;7:20–33.
LinkOut - more resources
Full Text Sources
Other Literature Sources
