MODUL-a multicenter randomized clinical trial of biomarker-driven maintenance therapy following first-line standard induction treatment of metastatic colorectal cancer: an adaptable signal-seeking approach
- PMID: 29644408
- PMCID: PMC11813285
- DOI: 10.1007/s00432-018-2632-6
MODUL-a multicenter randomized clinical trial of biomarker-driven maintenance therapy following first-line standard induction treatment of metastatic colorectal cancer: an adaptable signal-seeking approach
Abstract
Purpose: The old approach of one therapeutic for all patients with mCRC is evolving with a need to target specific molecular aberrations or cell-signalling pathways. Molecular screening approaches and new biomarkers are required to fully characterize tumours, identify patients most likely to benefit, and predict treatment response.
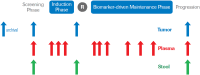
Methods: MODUL is a signal-seeking trial with a design that is highly adaptable, permitting modification of different treatment cohorts and inclusion of further additional cohorts based on novel evidence on new compounds/combinations that emerge during the study.
Results: MODUL is ongoing and its adaptable nature permits timely and efficient recruitment of patients into the most appropriate cohort. Recruitment will take place over approximately 5 years in Europe, Asia, Africa, and South America. The design of MODUL with ongoing parallel/sequential treatment cohorts means that the overall size and duration of the trial can be modified/prolonged based on accumulation of new data.
Conclusions: The early success of the current trial suggests that the design may provide definitive leads in a patient-friendly and relatively economical trial structure. Along with other biomarker-driven trials that are currently underway, it is hoped that MODUL will contribute to the continuing evolution of clinical trial design and permit a more 'tailored' approach to the treatment of patients with mCRC.
Keywords: Biomarker; FOLFOX + bevacizumab; MODUL; Metastatic colorectal cancer; Signal seeking; Switch maintenance therapy.
Conflict of interest statement
Ivan Bosanac, Belguendouz Bendahmane, and Christoph Mancao are employees of the study sponsor (F. Hoffmann-La Roche). All other authors report no relevant conflict of interest.
Figures

References
-
- Barker AD, Sigman CC, Kelloff GJ et al (2009) I-SPY2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther 86:97–100 - PubMed
-
- Bendell JC, Kim TW, Goh BC et al (2016) Clinical activity and safety of cobimetinib (cobi) and atezolizumab in colorectal cancer (CRC). J Clin Oncol 34 (suppl 4S; abstr 3502)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

