Defining Cell Identity with Single-Cell Omics
- PMID: 29644800
- PMCID: PMC6175476
- DOI: 10.1002/pmic.201700312
Defining Cell Identity with Single-Cell Omics
Abstract
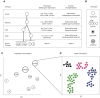
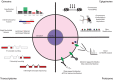
Cells are a fundamental unit of life, and the ability to study the phenotypes and behaviors of individual cells is crucial to understanding the workings of complex biological systems. Cell phenotypes (epigenomic, transcriptomic, proteomic, and metabolomic) exhibit dramatic heterogeneity between and within the different cell types and states underlying cellular functional diversity. Cell genotypes can also display heterogeneity throughout an organism, in the form of somatic genetic variation-most notably in the emergence and evolution of tumors. Recent technical advances in single-cell isolation and the development of omics approaches sensitive enough to reveal these aspects of cell identity have enabled a revolution in the study of multicellular systems. In this review, we discuss the technologies available to resolve the genomes, epigenomes, transcriptomes, proteomes, and metabolomes of single cells from a wide variety of living systems.
Keywords: epigenomics; genomics; proteomics; single-cell; technology; transcriptomics.
© 2018 The Authors. Proteomics Published by WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

References
-
- a) Beerman I., Bhattacharya D., Zandi S., Sigvardsson M., Weissman I. L., Bryder D., Rossi D. J., Proc. Natl. Acad. Sci. USA 2010, 107, 5465; - PMC - PubMed
- b) Challen G. A., Boles N. C., Chambers S. M., Goodell M. A., Cell Stem Cell 2010, 6, 265; - PMC - PubMed
- c) Kent D. G., Copley M. R., Benz C., Wohrer S., Dykstra B. J., Ma E., Cheyne J., Zhao Y., Bowie M. B., Zhao Y., Gasparetto M., Delaney A., Smith C., Marra M., Eaves C. J., Blood 2009, 113, 6342; - PubMed
- d) Kiel M. J., Yilmaz O. H., Iwashita T., Yilmaz O. H., Terhorst C., Morrison S. J., Cell 2005, 121, 1109; - PubMed
- e) Morita Y., Ema H., Nakauchi H., J. Exp. Med. 2010, 207, 1173. - PMC - PubMed
-
- Macaulay I. C., Haerty W., Kumar P., Li Y. I., Hu T. X., Teng M. J., Goolam M., Saurat N., Coupland P., Shirley L. M., Smith M., Van der Aa N., Banerjee R., Ellis P. D., Quail M. A., Swerdlow H. P., Zernicka‐Goetz M., Livesey F. J., Ponting C. P., Voet T., Nat. Methods 2015, 12, 519. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

