Fine Mapping the Interaction Between Dendritic Cell-Specific Intercellular Adhesion Molecule (ICAM)-3-Grabbing Nonintegrin and the Cytomegalovirus Envelope Glycoprotein B
- PMID: 29648611
- PMCID: PMC6049025
- DOI: 10.1093/infdis/jiy194
Fine Mapping the Interaction Between Dendritic Cell-Specific Intercellular Adhesion Molecule (ICAM)-3-Grabbing Nonintegrin and the Cytomegalovirus Envelope Glycoprotein B
Abstract
Background: Human cytomegalovirus (HCMV) is a leading cause of virally induced congenital disorders and morbidities in immunocompromised individuals, ie, transplant, cancer, or acquired immune deficiency syndrome patients. Human cytomegalovirus infects virtually all cell types through the envelope glycoprotein complex gH/gL/gO with or without a contribution of the pentameric gH/gL/pUL128L. Together with gH/gL, the HCMV envelope glycoprotein B (gB) contributes to the viral fusion machinery.
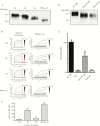
Methods: We previously showed that gB is a ligand for the C-type lectin dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) contributing to HCMV attachment to and infection of DC-SIGN-expressing cells. However, the features of the DC-SIGN/gB interaction remain unclear. To address this point, the role of glycans on gB and the consequences of mutagenesis and antibody-mediated blockades on both partners were examined in this study.
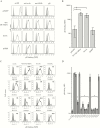
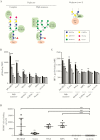
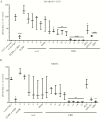
Results: We identified DC-SIGN amino acid residues involved in this interaction through an extensive mutagenesis study. We also showed the importance of high-mannose N-glycans decorating the asparagine residue at position 208, demonstrating that the antigenic domain 5 on gB is involved in the interaction with DC-SIGN. Finally, antibody-mediated blockades allowed us to identify DC-SIGN as a major HCMV attachment receptor on monocyte-derived dendritic cells.
Conclusions: Taken together, these results have permitted us to fine-map the interaction between DC-SIGN and HCMV gB.
Figures


Similar articles
-
Unprecedented Thiacalixarene Fucoclusters as Strong Inhibitors of Ebola cis-Cell Infection and HCMV-gB Glycoprotein/DC-SIGN C-type Lectin Interaction.Bioconjug Chem. 2019 Apr 17;30(4):1114-1126. doi: 10.1021/acs.bioconjchem.9b00066. Epub 2019 Apr 4. Bioconjug Chem. 2019. PMID: 30912645
-
Interaction of HIV-1 with dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin-expressing cells is influenced by gp120 envelope modifications associated with disease progression.FEBS J. 2006 Nov;273(21):4944-58. doi: 10.1111/j.1742-4658.2006.05491.x. Epub 2006 Sep 28. FEBS J. 2006. PMID: 17010165
-
N-Glycans on the Rift Valley Fever Virus Envelope Glycoproteins Gn and Gc Redundantly Support Viral Infection via DC-SIGN.Viruses. 2016 May 23;8(5):149. doi: 10.3390/v8050149. Viruses. 2016. PMID: 27223297 Free PMC article.
-
A fatal attraction: Mycobacterium tuberculosis and HIV-1 target DC-SIGN to escape immune surveillance.Trends Mol Med. 2003 Apr;9(4):153-9. doi: 10.1016/s1471-4914(03)00027-3. Trends Mol Med. 2003. PMID: 12727141 Review.
-
Role of the C-type lectins DC-SIGN and L-SIGN in Leishmania interaction with host phagocytes.Immunobiology. 2005;210(2-4):185-93. doi: 10.1016/j.imbio.2005.05.013. Immunobiology. 2005. PMID: 16164025 Free PMC article. Review.
Cited by
-
Association between single nucleotide polymorphisms and viral load in congenital cytomegalovirus infection.J Mother Child. 2021 Jul 16;24(4):9-17. doi: 10.34763/jmotherandchild.20202404.d-20-00014. J Mother Child. 2021. PMID: 33656306 Free PMC article.
-
Dendritic Cells and SARS-CoV-2 Infection: Still an Unclarified Connection.Cells. 2020 Sep 8;9(9):2046. doi: 10.3390/cells9092046. Cells. 2020. PMID: 32911691 Free PMC article. Review.
-
Non-permissive human conventional CD1c+ dendritic cells enable trans-infection of human primary renal tubular epithelial cells and protect BK polyomavirus from neutralization.PLoS Pathog. 2021 Feb 16;17(2):e1009042. doi: 10.1371/journal.ppat.1009042. eCollection 2021 Feb. PLoS Pathog. 2021. PMID: 33592065 Free PMC article.
-
Association between single nucleotide polymorphisms (SNPs) of IL1, IL12, IL28 and TLR4 and symptoms of congenital cytomegalovirus infection.PLoS One. 2020 May 18;15(5):e0233096. doi: 10.1371/journal.pone.0233096. eCollection 2020. PLoS One. 2020. PMID: 32421725 Free PMC article.
-
Single Nucleotide Polymorphisms of Interleukins and Toll-like Receptors and Neuroimaging Results in Newborns with Congenital HCMV Infection.Viruses. 2021 Sep 7;13(9):1783. doi: 10.3390/v13091783. Viruses. 2021. PMID: 34578364 Free PMC article.
References
-
- Dioverti MV, Razonable RR. Cytomegalovirus. Microbiol Spectr 2016; 4:Aug; 4(4). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

