The root-knot nematode Meloidogyne incognita produces a functional mimic of the Arabidopsis INFLORESCENCE DEFICIENT IN ABSCISSION signaling peptide
- PMID: 29648636
- PMCID: PMC5972575
- DOI: 10.1093/jxb/ery135
The root-knot nematode Meloidogyne incognita produces a functional mimic of the Arabidopsis INFLORESCENCE DEFICIENT IN ABSCISSION signaling peptide
Abstract
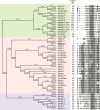
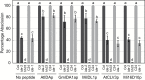
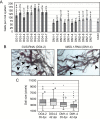
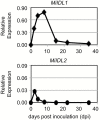
INFLORESCENCE DEFICIENT IN ABSCISSION (IDA) is a signaling peptide that regulates cell separation in Arabidopsis including floral organ abscission and lateral root emergence. IDA is highly conserved in dicotyledonous flowering plant genomes. IDA-like sequences were also found in the genomic sequences of root-knot nematodes, Meloidogyne spp., which are globally deleterious pathogens of agriculturally important plants, but the role of these genes is unknown. Exogenous treatment of the Arabidopsis ida mutant with synthetic peptide identical to the M. incognita IDA-like 1 (MiIDL1) protein sequence minus its N-terminal signal peptide recovered both the abscission and root architecture defects. Constitutive expression of the full-length MiIDL1 open reading frame in the ida mutant substantially recovered the delayed floral organ abscission phenotype whereas transformants expressing a construct missing the MiIDL1 signal peptide retained the delayed abscission phenotype. Importantly, wild-type Arabidopsis plants harboring an MiIDL1-RNAi construct and infected with nematodes had approximately 40% fewer galls per root than control plants. Thus, the MiIDL1 gene produces a functional IDA mimic that appears to play a role in successful gall development on Arabidopsis roots.
Figures


Comment in
-
Nematode-secreted peptides and host factor mimicry.J Exp Bot. 2018 May 25;69(12):2866-2868. doi: 10.1093/jxb/ery144. J Exp Bot. 2018. PMID: 29846675 Free PMC article.
References
-
- Alkharouf NW, Klink VP, Chouikha IB, Beard HS, MacDonald MH, Meyer S, Knap HT, Khan R, Matthews BF. 2006. Timecourse microarray analyses reveal global changes in gene expression of susceptible Glycine max (soybean) roots during infection by Heterodera glycines (soybean cyst nematode). Planta 224, 838–852. - PubMed
-
- Barton MK. 2010. Twenty years on: the inner workings of the shoot apical meristem, a developmental dynamo. Developmental Biology 341, 95–113. - PubMed
-
- Bobay BG, DiGennaro P, Scholl E, Imin N, Djordjevic MA, Mck Bird D. 2013. Solution NMR studies of the plant peptide hormone CEP inform function. FEBS Letters 587, 3979–3985. - PubMed
-
- Branch C, Hwang CF, Navarre DA, Williamson VM. 2004. Salicylic acid is part of the Mi-1-mediated defense response to root-knot nematode in tomato. Molecular Plant-Microbe Interactions 17, 351–356. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

