Glibenclamide Produces Region-Dependent Effects on Cerebral Edema in a Combined Injury Model of Traumatic Brain Injury and Hemorrhagic Shock in Mice
- PMID: 29648981
- PMCID: PMC6098411
- DOI: 10.1089/neu.2016.4696
Glibenclamide Produces Region-Dependent Effects on Cerebral Edema in a Combined Injury Model of Traumatic Brain Injury and Hemorrhagic Shock in Mice
Abstract
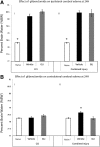
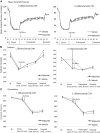
Cerebral edema is critical to morbidity/mortality in traumatic brain injury (TBI) and is worsened by hypotension. Glibenclamide may reduce cerebral edema by inhibiting sulfonylurea receptor-1 (Sur1); its effect on diffuse cerebral edema exacerbated by hypotension/resuscitation is unknown. We aimed to determine if glibenclamide improves pericontusional and/or diffuse edema in controlled cortical impact (CCI) (5m/sec, 1 mm depth) plus hemorrhagic shock (HS) (35 min), and compare its effects in CCI alone. C57BL/6 mice were divided into five groups (n = 10/group): naïve, CCI+vehicle, CCI+glibenclamide, CCI+HS+vehicle, and CCI+HS+glibenclamide. Intravenous glibenclamide (10 min post-injury) was followed by a subcutaneous infusion for 24 h. Brain edema in injured and contralateral hemispheres was subsequently quantified (wet-dry weight). This protocol brain water (BW) = 80.4% vehicle vs. 78.3% naïve, p < 0.01) but was not reduced by glibenclamide (I%BW = 80.4%). Ipsilateral edema also developed in CCI alone (I%BW = 80.2% vehicle vs. 78.3% naïve, p < 0.01); again unaffected by glibenclamide (I%BW = 80.5%). Contralateral (C) %BW in CCI+HS was increased in vehicle (78.6%) versus naive (78.3%, p = 0.02) but unchanged in CCI (78.3%). At 24 h, glibenclamide treatment in CCI+HS eliminated contralateral cerebral edema (C%BW = 78.3%) with no difference versus naïve. By 72 h, contralateral cerebral edema had resolved (C%BW = 78.5 ± 0.09% vehicle vs. 78.3 ± 0.05% naïve). Glibenclamide decreased 24 h contralateral cerebral edema in CCI+HS. This beneficial effect merits additional exploration in the important setting of TBI with polytrauma, shock, and resuscitation. Contralateral edema did not develop in CCI alone. Surprisingly, 24 h of glibenclamide treatment failed to decrease ipsilateral edema in either model. Interspecies dosing differences versus prior studies may play an important role in these findings. Mechanisms underlying brain edema may differ regionally, with pericontusional/osmolar swelling refractory to glibenclamide but diffuse edema (via Sur1) from combined injury and/or resuscitation responsive to this therapy. TBI phenotype may mandate precision medicine approaches to treat brain edema.
Keywords: HS; Sur1; TBI; cerebral edema; glibenclamide/glyburide.
Conflict of interest statement
B.J.M. received research grants from Remedy Pharmaceuticals for clinical trial activities and was a site principal investigator for “Glyburide Advantage in Malignant Edema and Stroke (GAMES-RP)” a Remedy Pharmaceuticals funded study of glibenclamide for malignant edema in stroke. The other authors have nothing to disclose.
Figures


Similar articles
-
Precision Effects of Glibenclamide on MRI Endophenotypes in Clinically Relevant Murine Traumatic Brain Injury.Crit Care Med. 2023 Feb 1;51(2):e45-e59. doi: 10.1097/CCM.0000000000005749. Epub 2022 Dec 16. Crit Care Med. 2023. PMID: 36661464 Free PMC article.
-
Glibenclamide Treatment in Traumatic Brain Injury: Operation Brain Trauma Therapy.J Neurotrauma. 2021 Mar;38(5):628-645. doi: 10.1089/neu.2020.7421. Epub 2020 Dec 18. J Neurotrauma. 2021. PMID: 33203303 Free PMC article.
-
MRI assessment of cerebral blood flow after experimental traumatic brain injury combined with hemorrhagic shock in mice.J Cereb Blood Flow Metab. 2013 Jan;33(1):129-36. doi: 10.1038/jcbfm.2012.145. Epub 2012 Oct 17. J Cereb Blood Flow Metab. 2013. PMID: 23072750 Free PMC article.
-
Role of Sulfonylurea Receptor 1 and Glibenclamide in Traumatic Brain Injury: A Review of the Evidence.Int J Mol Sci. 2020 Jan 9;21(2):409. doi: 10.3390/ijms21020409. Int J Mol Sci. 2020. PMID: 31936452 Free PMC article. Review.
-
Glibenclamide in cerebral ischemia and stroke.Neurocrit Care. 2014 Apr;20(2):319-33. doi: 10.1007/s12028-013-9923-1. Neurocrit Care. 2014. PMID: 24132564 Free PMC article. Review.
Cited by
-
BIIB093 (IV glibenclamide): an investigational compound for the prevention and treatment of severe cerebral edema.Expert Opin Investig Drugs. 2019 Dec;28(12):1031-1040. doi: 10.1080/13543784.2019.1681967. Epub 2019 Oct 24. Expert Opin Investig Drugs. 2019. PMID: 31623469 Free PMC article. Review.
-
Research progress on pleiotropic neuroprotective drugs for traumatic brain injury.Front Pharmacol. 2023 Jul 5;14:1185533. doi: 10.3389/fphar.2023.1185533. eCollection 2023. Front Pharmacol. 2023. PMID: 37475717 Free PMC article. Review.
-
Pathophysiological Responses and Roles of Astrocytes in Traumatic Brain Injury.Int J Mol Sci. 2021 Jun 15;22(12):6418. doi: 10.3390/ijms22126418. Int J Mol Sci. 2021. PMID: 34203960 Free PMC article. Review.
-
Choice of Whole Blood versus Lactated Ringer's Resuscitation Modifies the Relationship between Blood Pressure Target and Functional Outcome after Traumatic Brain Injury plus Hemorrhagic Shock in Mice.J Neurotrauma. 2021 Oct 15;38(20):2907-2917. doi: 10.1089/neu.2021.0157. Epub 2021 Sep 15. J Neurotrauma. 2021. PMID: 34269621 Free PMC article.
-
Multifaceted Benefit of Whole Blood Versus Lactated Ringer's Resuscitation After Traumatic Brain Injury and Hemorrhagic Shock in Mice.Neurocrit Care. 2021 Jun;34(3):781-794. doi: 10.1007/s12028-020-01084-1. Epub 2020 Sep 4. Neurocrit Care. 2021. PMID: 32886294 Free PMC article.
References
-
- Faul M., and Coronado V. (2015). Epidemiology of traumatic brain injury. Handb. Clin. Neurol. 127, 3–13 - PubMed
-
- Langlois J.A., Rutland-Brown W., and Wald M.M. (2006). The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 21, 375–378 - PubMed
-
- Ghajar J. (2000). Traumatic brain injury. Lancet 356, 923–929 - PubMed
-
- Chestnut R.M. (1995). Secondary brain insults after head injury: clinical perspectives. New Horiz. 3, 366–375 - PubMed
-
- Tisherman S.A., Schmicker R.H., Brasel K.J., Bulger E.M., Kerby J.D., Minei J.P., Powell J.L., Reiff D.A., Rizoli S.B., and Schreiber M.A. (2015). Detailed description of all deaths in both the shock and traumatic brain injury hypertonic saline trials of the Resuscitation Outcomes Consortium. Ann. Surg. 261, 586–590 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

