Coordination of GPR40 and Ketogenesis Signaling by Medium Chain Fatty Acids Regulates Beta Cell Function
- PMID: 29649104
- PMCID: PMC5946258
- DOI: 10.3390/nu10040473
Coordination of GPR40 and Ketogenesis Signaling by Medium Chain Fatty Acids Regulates Beta Cell Function
Abstract
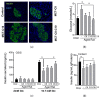
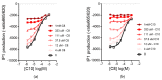
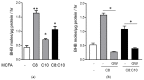
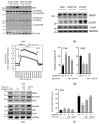
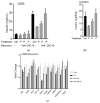
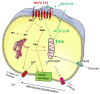
Diabetes prevalence increases with age, and β-cell dysfunction contributes to the incidence of the disease. Dietary lipids have been recognized as contributory factors in the development and progression of the disease. Unlike long chain triglycerides, medium chain triglycerides (MCT) increase fat burning in animal and human subjects as well as serum C-peptide in type 2 diabetes patients. We evaluated the beneficial effects of MCT on β-cells in vivo and in vitro. MCT improved glycemia in aged rats via β-cell function assessed by measuring insulin secretion and content. In β-cells, medium chain fatty acid (MCFA)-C10 activated fatty acid receptor 1 FFAR1/GPR40, while MCFA-C8 induced mitochondrial ketogenesis and the C8:C10 mixture improved β cell function. We showed that GPR40 signaling positively impacts ketone body production in β-cells, and chronic treatment with β-hydroxybutyrate (BHB) improves β-cell function. We also showed that BHB and MCFA help β-cells recover from lipotoxic stress by improving mitochondrial function and increasing the expression of genes involved in β-cell function and insulin biogenesis, such as Glut2, MafA, and NeuroD1 in primary human islets. MCFA offers a therapeutic advantage in the preservation of β-cell function as part of a preventative strategy against diabetes in at risk populations.
Keywords: BHB; GPR40; insulin secretion; ketogenesis; lipotoxicity; medium chain fatty acid; mitochondria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Cowie C.C., Rust K.F., Byrd-Holt D.D., Eberhardt M.S., Flegal K.M., Engelgau M.M., Saydah S.H., Williams D.E., Geiss L.S., Gregg E.W. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999–2002. Diabetes Care. 2006;29:1263–1268. doi: 10.2337/dc06-0062. - DOI - PubMed
-
- Cheng Y.J., Gregg E.W., Geiss L.S., Imperatore G., Williams D.E., Zhang X., Albright A.L., Cowie C.C., Klein R., Saaddine J.B. Association of A1C and fasting plasma glucose levels with diabetic retinopathy prevalence in the U.S. population: Implications for diabetes diagnostic thresholds. Diabetes Care. 2009;32:2027–2032. doi: 10.2337/dc09-0440. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

