Nanocoatings for Chronic Wound Repair-Modulation of Microbial Colonization and Biofilm Formation
- PMID: 29649179
- PMCID: PMC5979353
- DOI: 10.3390/ijms19041179
Nanocoatings for Chronic Wound Repair-Modulation of Microbial Colonization and Biofilm Formation
Abstract
Wound healing involves a complex interaction between immunity and other natural host processes, and to succeed it requires a well-defined cascade of events. Chronic wound infections can be mono- or polymicrobial but their major characteristic is their ability to develop a biofilm. A biofilm reduces the effectiveness of treatment and increases resistance. A biofilm is an ecosystem on its own, enabling the bacteria and the host to establish different social interactions, such as competition or cooperation. With an increasing incidence of chronic wounds and, implicitly, of chronic biofilm infections, there is a need for alternative therapeutic agents. Nanotechnology shows promising openings, either by the intrinsic antimicrobial properties of nanoparticles or their function as drug carriers. Nanoparticles and nanostructured coatings can be active at low concentrations toward a large variety of infectious agents; thus, they are unlikely to elicit emergence of resistance. Nanoparticles might contribute to the modulation of microbial colonization and biofilm formation in wounds. This comprehensive review comprises the pathogenesis of chronic wounds, the role of chronic wound colonization and infection in the healing process, the conventional and alternative topical therapeutic approaches designed to combat infection and stimulate healing, as well as revolutionizing therapies such as nanotechnology-based wound healing approaches.
Keywords: antimicrobial nanoparticles; biofilm formation; chronic wound; nanocoatings; tolerance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


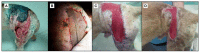
References
-
- Demidova-Rice T.N., Hamblin M.R., Herman I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Skin Wound Care. 2012;25:304–314. doi: 10.1097/01.ASW.0000416006.55218.d0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

