Carbapenem-resistant Gram-negative pathogens in a German university medical center: Prevalence, clinical implications and the role of novel β-lactam/β-lactamase inhibitor combinations
- PMID: 29649276
- PMCID: PMC5896976
- DOI: 10.1371/journal.pone.0195757
Carbapenem-resistant Gram-negative pathogens in a German university medical center: Prevalence, clinical implications and the role of novel β-lactam/β-lactamase inhibitor combinations
Abstract
Objectives: To determine the spectrum of infections with multidrug-resistant Gram-negative bacteria (MDR-GNB) and the clinical impact of the newly available betalactam/betalactamase inhibitor combinations ceftolozane/tazobactam and ceftazidime/avibactam in a German academic tertiary care center.
Methods: Retrospective analysis.
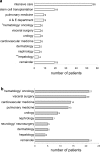
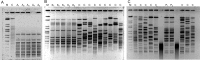
Results: Between September 1, 2015 and August 31, 2016, 119 individual patients (0.22% of all hospital admissions) were colonized or infected with carbapenem-resistant MDR-GNB. The species distribution was Pseudomonas aeruginosa, n = 66; Enterobacteriaceae spp., n = 44; and Acinetobacter baumannii, n = 18. In 9 patients, carbapenem-resistant isolates belonging to more than one species were detected. Infection was diagnosed in 50 patients (total: 42.0%; nosocomial pneumonia: n = 23, 19.3%; bloodstream infection: n = 11, 9.2%). Antimicrobial treatment with broad-spectrum antibiotics prior to detection of a carbapenem-resistant isolate was documented in 105 patients (88.2%, prior administration of carbapenems: 62.2%). Nosocomial transmission was documented in 29 patients (24.4%). In 26 patients (21.8%), at least one carbapenem-susceptible, third-generation cephalosporin non-susceptible isolate was documented prior to detection of a carbapenem-resistant isolate belonging to the same species (median 38 days, IQR 23-78). 12 patients (10.1%) had documented previous contact to the healthcare system in a country with high burden of carbapenemase-producing strains. Genes encoding carbapenemases were detected in 60/102 patient isolates (58.8%; VIM-2, n = 25; OXA-48, n = 21; OXA-23-like, n = 10). Susceptibility to colistin was 94.3%. Ceftolozane/tazobactam and ceftazidime/avibactam were administered to 3 and 5 patients, respectively (in-hospital mortality: 66% and 100%). Development of drug-resistance under therapy was observed for both antimicrobials.
Conclusions: i) The major predisposing factors for acquisition of carbapenem-resistant MDR-GNB were selective pressure due to preceding antimicrobial therapy and nosocomial transmission. ii) Colistin remains the backbone of antimicrobial chemotherapy for infections caused by carbapenem-resistant MDR-GNB. iii) Novel β-lactam/β-lactamase inhibitor combinations are of limited usefulness in our setting because of the high prevalence of Ambler class B carbapenemases and the emergence of nonsusceptibility under therapy.
Conflict of interest statement
Figures
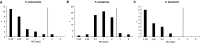
References
-
- Grundmann H, Glasner C, Albiger B, Aanensen DM, Tomlinson CT, Andrasević AT, et al. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): a prospective, multinational study. Lancet Infect Dis. 2017;17: 153–163. doi: 10.1016/S1473-3099(16)30257-2 - DOI - PubMed
-
- Perez F, El Chakhtoura NG, Papp-Wallace KM, Wilson BM, Bonomo RA. Treatment options for infections caused by carbapenem-resistant Enterobacteriaceae: can we apply “precision medicine” to antimicrobial chemotherapy? Expert Opin Pharmacother. 2016;17: 761–81. doi: 10.1517/14656566.2016.1145658 - DOI - PMC - PubMed
-
- Falagas ME, Lourida P, Poulikakos P, Rafailidis PI, Tansarli GS. Antibiotic treatment of infections due to carbapenem-resistant Enterobacteriaceae: systematic evaluation of the available evidence. Antimicrob Agents Chemother. 2014;58: 654–63. doi: 10.1128/AAC.01222-13 - DOI - PMC - PubMed
-
- Livermore DM, Mushtaq S, Meunier D, Hopkins KL, Hill R, Adkin R, et al. Activity of ceftolozane/tazobactam against surveillance and “problem” Enterobacteriaceae, Pseudomonas aeruginosa and non-fermenters from the British Isles. J Antimicrob Chemother. 2017; doi: 10.1093/jac/dkx136 - DOI - PMC - PubMed
-
- Carmeli Y, Armstrong J, Laud PJ, Newell P, Stone G, Wardman A, et al. Ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): a randomised, pathogen-directed,. Lancet Infect Dis. 2016;16: 661–73. doi: 10.1016/S1473-3099(16)30004-4 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

