The evolution of nucleoside analogue antivirals: A review for chemists and non-chemists. Part 1: Early structural modifications to the nucleoside scaffold
- PMID: 29649496
- PMCID: PMC6396324
- DOI: 10.1016/j.antiviral.2018.04.004
The evolution of nucleoside analogue antivirals: A review for chemists and non-chemists. Part 1: Early structural modifications to the nucleoside scaffold
Abstract
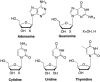
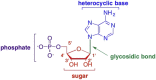
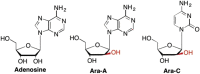
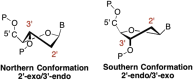
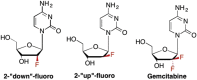
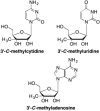
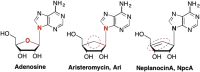
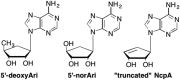
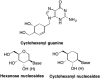
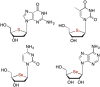
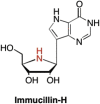
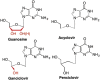
This is the first of two invited articles reviewing the development of nucleoside-analogue antiviral drugs, written for a target audience of virologists and other non-chemists, as well as chemists who may not be familiar with the field. Rather than providing a simple chronological account, we have examined and attempted to explain the thought processes, advances in synthetic chemistry and lessons learned from antiviral testing that led to a few molecules being moved forward to eventual approval for human therapies, while others were discarded. The present paper focuses on early, relatively simplistic changes made to the nucleoside scaffold, beginning with modifications of the nucleoside sugars of Ara-C and other arabinose-derived nucleoside analogues in the 1960's. A future paper will review more recent developments, focusing especially on more complex modifications, particularly those involving multiple changes to the nucleoside scaffold. We hope that these articles will help virologists and others outside the field of medicinal chemistry to understand why certain drugs were successfully developed, while the majority of candidate compounds encountered barriers due to low-yielding synthetic routes, toxicity or other problems that led to their abandonment.
Keywords: Analogue; Anticancer; Antiviral; History; Modification; Nucleoside.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures




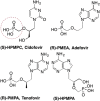
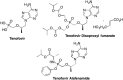
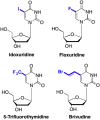
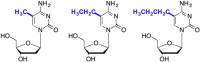

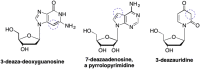

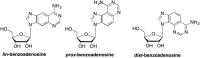

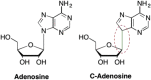
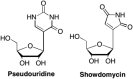


References
-
- Agrofoglio L., Suhas E., Farese A., Condom R., Challand S.R., Earl R.A., Guedj R. Synthesis of carbocyclic nucleosides. Tetrahedron. 1994;50:10611–10670.
-
- Aihong K., Joon H.H. Synthesis and antiviral activity of C-fluoro-branched cyclopropyl nucleosides. Eur. J. Med. Chem. 2007;42:487–493. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

