Mesoporous silicate nanoparticles/3D nanofibrous scaffold-mediated dual-drug delivery for bone tissue engineering
- PMID: 29649529
- PMCID: PMC5972066
- DOI: 10.1016/j.jconrel.2018.04.011
Mesoporous silicate nanoparticles/3D nanofibrous scaffold-mediated dual-drug delivery for bone tissue engineering
Abstract
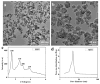
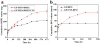
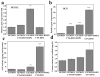
Controlled delivery systems play a critical role in the success of bone morphogenetic proteins (i.e., BMP2 and BMP7) for challenged bone repair. Instead of single-drug release that is currently and commonly prevalent, dual-drug delivery strategies are highly desired to achieve effective bone regeneration because natural bone repair process is driven by multiple factors. Particularly, angiogenesis is essential for osteogenesis and requires more than just one factor (e.g., Vascular Endothelial Growth Factor, VEGF). Therefore, we developed a novel mesoporous silicate nanoparticles (MSNs) incorporated-3D nanofibrous gelatin (GF) scaffold for dual-delivery of BMP2 and deferoxamine (DFO). DFO is a hypoxia-mimetic drug that can activate hypoxia-inducible factor-1 alpha (HIF-1α), and trigger subsequent angiogenesis. Sustained BMP2 release system was achieved through encapsulation into large-pored MSNs, while the relative short-term release of DFO was engineered through covalent conjugation with chitosan to reduce its cytotoxicity and elongate its half-life. Both MSNs and DFO were incorporated onto a porous 3D GF scaffold to serve as a biomimetic osteogenic microenvironment. Our data indicated that DFO and BMP2 were released from a scaffold at different release rates (10 vs 28 days) yet maintained their angiogenic and osteogenic ability, respectively. Importantly, our data indicated that the released DFO significantly improved BMP2-induced osteogenic differentiation where the dose/duration was important for its effects in both mouse and human stem cell models. Thus, we developed a novel and tunable MSNs/GF 3D scaffold-mediated dual-drug delivery system and studied the potential application of the both FDA-approved DFO and BMP2 for bone tissue engineering.
Keywords: Angiogenesis; Deferoxamine; Dual release system; Mesoporous silicate nanoparticles; Nanofibrous scaffold; Osteogenesis.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures






References
-
- Krishnakumar GS, Roffi A, Reale D, Kon E, Filardo G. Clinical application of bone morphogenetic proteins for bone healing: a systematic review. Int Orthop. 2017:1–11. - PubMed
-
- Lad SP, Bagley JH, Karikari IO, Babu R, Ugiliweneza B, Kong M, Isaacs RE, Bagley CA, Gottfried ON, Patil CG. Cancer after spinal fusion: the role of bone morphogenetic protein. Neurosurgery. 2013;73:440–449. - PubMed
-
- Gothard D, Smith E, Kanczler J, Rashidi H, Qutachi O, Henstock J, Rotherham M, El Haj A, Shakesheff K, Oreffo R. Tissue engineered bone using select growth factors: a comprehensive review of animal studies and clinical translation studies in man. Eur Cells Mater. 2014;28:166–208. - PubMed
-
- Biondi M, Ungaro F, Quaglia F, Netti PA. Controlled drug delivery in tissue engineering. Adv Drug Deliver Rev. 2008;60:229–242. - PubMed
-
- Bessa PC, Casal M, Reis R. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery) J Tissue Eng Regen Med. 2008;2:81–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

