Autophagic-related cell death of Trypanosoma brucei induced by bacteriocin AS-48
- PMID: 29649664
- PMCID: PMC6039360
- DOI: 10.1016/j.ijpddr.2018.03.002
Autophagic-related cell death of Trypanosoma brucei induced by bacteriocin AS-48
Abstract
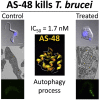
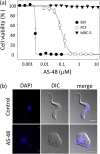
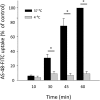
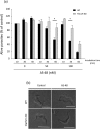
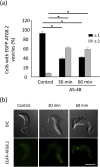
The parasitic protozoan Trypanosoma brucei is the causative agent of human African trypanosomiasis (sleeping sickness) and nagana. Current drug therapies have limited efficacy, high toxicity and/or are continually hampered by the appearance of resistance. Antimicrobial peptides have recently attracted attention as potential parasiticidal compounds. Here, we explore circular bacteriocin AS-48's ability to kill clinically relevant bloodstream forms of T. brucei gambiense, T. brucei rhodesiense and T. brucei brucei. AS-48 exhibited excellent anti-trypanosomal activity in vitro (EC50 = 1-3 nM) against the three T. brucei subspecies, but it was innocuous to human cells at 104-fold higher concentrations. In contrast to its antibacterial action, AS-48 does not kill the parasite through plasma membrane permeabilization but by targeting intracellular compartments. This was evidenced by the fact that vital dye internalization-prohibiting concentrations of AS-48 could kill the parasite at 37 °C but not at 4 °C. Furthermore, AS-48 interacted with the surface of the parasite, at least in part via VSG, its uptake was temperature-dependent and clathrin-depleted cells were less permissive to the action of AS-48. The bacteriocin also caused the appearance of myelin-like structures and double-membrane autophagic vacuoles. These changes in the parasite's ultrastructure were confirmed by fluorescence microscopy as AS-48 induced the production of EGFP-ATG8.2-labeled autophagosomes. Collectively, these results indicate AS-48 kills the parasite through a mechanism involving clathrin-mediated endocytosis of VSG-bound AS-48 and the induction of autophagic-like cell death. As AS-48 has greater in vitro activity than the drugs currently used to treat T. brucei infection and does not present any signs of toxicity in mammalian cells, it could be an attractive lead compound for the treatment of sleeping sickness and nagana.
Keywords: AS-48; Antimicrobial peptides; Autophagy; Sleeping sickness; Trypanocidal drugs; Trypanosoma brucei.
Copyright © 2018 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures



References
-
- Ananou S., Muñoz A., Gálvez A., Martínez-Bueno M., Maqueda M., Valdivia E. Optimization of enterocin AS-48 production on a whey-based substrate. Int. Dairy J. 2008;18:923–927. doi: 10.1016/j.idairyj.2008.02.001. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

