Effectiveness of ultrasonography and nerve conduction studies in the diagnosing of carpal tunnel syndrome: clinical trial on accuracy
- PMID: 29649998
- PMCID: PMC5898048
- DOI: 10.1186/s12891-018-2036-4
Effectiveness of ultrasonography and nerve conduction studies in the diagnosing of carpal tunnel syndrome: clinical trial on accuracy
Abstract
Background: The aim of this study was to evaluate the effectiveness of two diagnostic tests routinely used for diagnosing carpal tunnel syndrome (CTS)-ultrasonography (US) and nerve conduction studies (NCS)-by comparing their accuracy based on surgical results, with the remission of paresthesia as the reference standard.
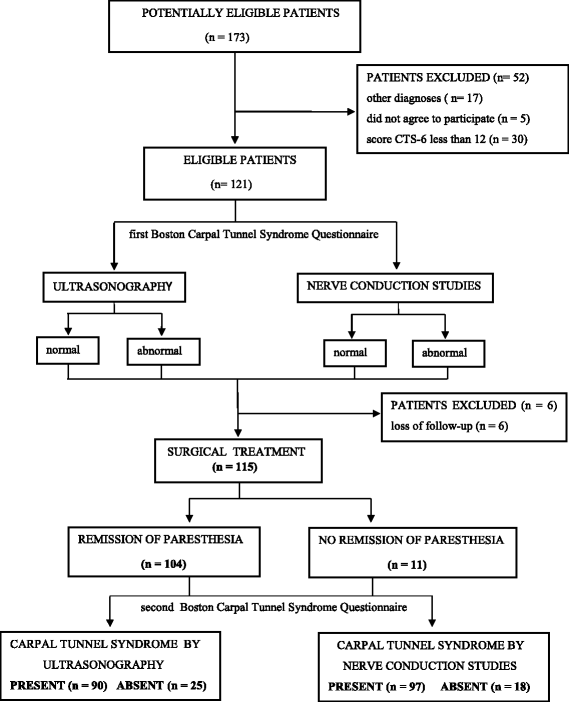
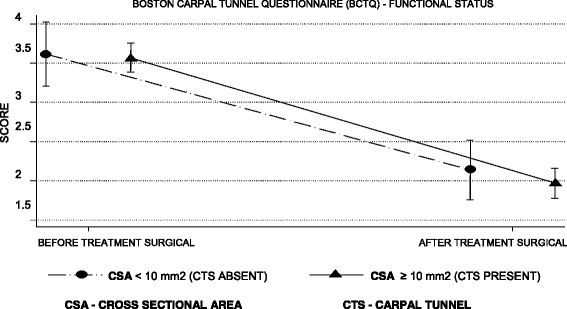
Methods: We enrolled 115 patients, all of the female gender with a high probability of a clinical diagnosis of CTS. All patients underwent US and NCS for a diagnosis and subsequent surgical treatment. As a primary outcome, the accuracy of the US and NCS diagnoses was measured by comparing their diagnoses compared with those determined by the surgical outcomes. Their accuracy was secondarily evaluated based on before and after scores of the Boston Carpal Tunnel Questionnaire (BCTQ).
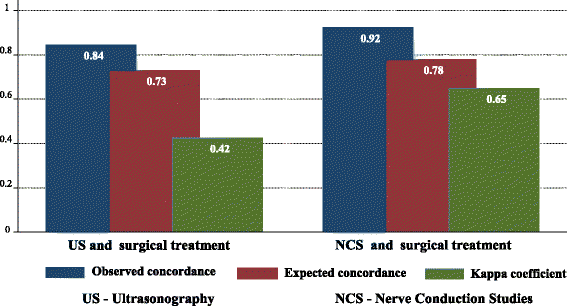
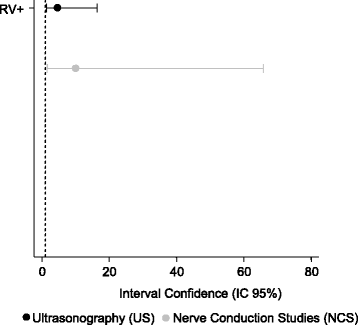
Results: Overall, 104 patients (90.4%) were diagnosed with CTS by the surgical reference standard, 97 (84.3%) by NCS, and 90 (78.3%) by US. The concordance of NCS and surgical treatment (p < 0.001; kappa = 0.648) was superior to that of US and surgical treatment (p < 0.001; kappa = 0.423). The sensitivity and specificity of US and NCS were similar (p = 1.000 and p = 0.152, respectively: McNemar's test). The BCTQ scores were lower after surgery in patients diagnosed by both US and NCS (p < 0.001and p < 0.001, respectively: analysis of variance).
Conclusions: US and NCS effectively diagnosed CTS with good sensitivity but were not effective enough to rule out a suspicion of CTS.
Trial registration: This study was registered at September, 10 th, 2015, and the registration number was NCT02553811 .
Keywords: Carpal tunnel syndrome; Clinical diagnosis; Diagnostic accuracy; Diagnostic practices; Electrodiagnostic testing; Electromyograph; Nerve conduction studies; Surgical treatment; Ultrasonography; Ultrasound.
Conflict of interest statement
Ethics approval and consent to participate
The Ethics and Research Committees of Federal University of São Paulo/Paulista School of Medicine, São Paulo, São Paulo State, Brazil (approval No. 244468) on 12 April 2013 and Paraiba Valley Regional Hospital and Taubaté University Hospital, University of Taubaté, Taubaté, São Paulo State, Brazil (No. 009/13) on 18 June 2013 approved this study. We have obtained the written informed consent for participation in the study from all participants.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
-
- Pimentel BF, Abicalaf CA, Braga L, et al. Cross-sectional area of the median nerve characterized by ultrasound in patients with carpal tunnel syndrome before and after release of the transverse carpal ligament. J Diagn Med Sonography. 2013;29:116–121. doi: 10.1177/8756479313477731. - DOI
-
- Graham B, Regehr G, Naglie G, et al. Development and validation of diagnostic criteria for carpal tunnel syndrome. J Hand Surg Am. 2006;31:919–924. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

