Treating cat allergy with monoclonal IgG antibodies that bind allergen and prevent IgE engagement
- PMID: 29650949
- PMCID: PMC5897525
- DOI: 10.1038/s41467-018-03636-8
Treating cat allergy with monoclonal IgG antibodies that bind allergen and prevent IgE engagement
Abstract
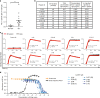
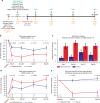
Acute allergic symptoms are caused by allergen-induced crosslinking of allergen-specific immunoglobulin E (IgE) bound to Fc-epsilon receptors on effector cells. Desensitization with allergen-specific immunotherapy (SIT) has been used for over a century, but the dominant protective mechanism remains unclear. One consistent observation is increased allergen-specific IgG, thought to competitively block allergen binding to IgE. Here we show that the blocking potency of the IgG response to Cat-SIT is heterogeneous. Next, using two potent, pre-selected allergen-blocking monoclonal IgG antibodies against the immunodominant cat allergen Fel d 1, we demonstrate that increasing the IgG/IgE ratio reduces the allergic response in mice and in cat-allergic patients: a single dose of blocking IgG reduces clinical symptoms in response to nasal provocation (ANCOVA, p = 0.0003), with a magnitude observed at day 8 similar to that reported with years of conventional SIT. This study suggests that simply augmenting the blocking IgG/IgE ratio may reverse allergy.
Conflict of interest statement
All authors are employees of Regeneron Pharmaceuticals, Inc, and may hold stock and/or stock options in the company.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

