Progress and challenges towards targeted delivery of cancer therapeutics
- PMID: 29650952
- PMCID: PMC5897557
- DOI: 10.1038/s41467-018-03705-y
Progress and challenges towards targeted delivery of cancer therapeutics
Abstract
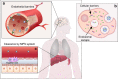
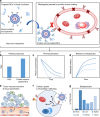
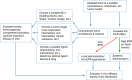
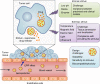
Targeted delivery approaches for cancer therapeutics have shown a steep rise over the past few decades. However, compared to the plethora of successful pre-clinical studies, only 15 passively targeted nanocarriers (NCs) have been approved for clinical use and none of the actively targeted NCs have advanced past clinical trials. Herein, we review the principles behind targeted delivery approaches to determine potential reasons for their limited clinical translation and success. We propose criteria and considerations that must be taken into account for the development of novel actively targeted NCs. We also highlight the possible directions for the development of successful tumor targeting strategies.
Conflict of interest statement
D.P. declares financial interest in Quiet Therapeutics, ART Biosciences, and SEPL pharma. J.M.K. holds equity in Alivio Therapeutics, a company that has an option to license IP generated by J.M.K. and that may benefit financially if the IP is licensed and further validated. The interests of J.M.K were reviewed and are subject to a management plan overseen by his institution in accordance with its conflict of interest policies. The remaining authors declare no competing interests.
Figures
References
-
- Wilhelm S, et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016;1:16014. doi: 10.1038/natrevmats.2016.14. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

