Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model
- PMID: 29651102
- PMCID: PMC5938041
- DOI: 10.1038/s12276-018-0058-5
Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model
Abstract
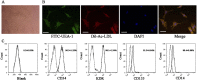
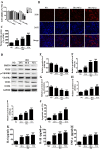
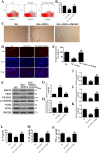
Diabetic foot ulcers (DFU) increase the risks of infection and amputation in patients with diabetes mellitus (DM). The impaired function and senescence of endothelial progenitor cells (EPCs) and high glucose-induced ROS likely exacerbate DFUs. We assessed EPCs in 60 patients with DM in a hospital or primary care setting. We also evaluated the therapeutic effects of exosomes secreted from adipose-derived stem cells (ADSCs) on stress-mediated senescence of EPCs induced by high glucose. Additionally, the effects of exosomes and Nrf2 overexpression in ADSCs were investigated in vitro and in vivo in a diabetic rat model. We found that ADSCs that secreted exosomes promoted proliferation and angiopoiesis in EPCs in a high glucose environment and that overexpression of Nrf2 increased this protective effect. Wounds in the feet of diabetic rats had a significantly reduced ulcerated area when treated with exosomes from ADSCs overexpressing Nrf2. Increased granulation tissue formation, angiogenesis, and levels of growth factor expression as well as reduced levels of inflammation and oxidative stress-related proteins were detected in wound beds. Our data suggest that exosomes from ADSCs can potentially promote wound healing, particularly when overexpressing Nrf2 and therefore that the transplantation of exosomes may be suitable for clinical application in the treatment of DFUs.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Fetterolf DE, Istwan NB, Stanziano GJ. An evaluation of healing metrics associated with commonly used advanced wound care products for the treatment of chronic diabetic foot ulcers. Manag. Care. 2014;23:31–38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

