Chemical probes and drug leads from advances in synthetic planning and methodology
- PMID: 29651105
- PMCID: PMC6707071
- DOI: 10.1038/nrd.2018.53
Chemical probes and drug leads from advances in synthetic planning and methodology
Abstract
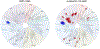
Screening of small-molecule libraries is a productive method for identifying both chemical probes of disease-related targets and potential starting points for drug discovery. In this article, we focus on strategies such as diversity-oriented synthesis that aim to explore novel areas of chemical space efficiently by populating small-molecule libraries with compounds containing structural features that are typically under-represented in commercially available screening collections. Drawing from more than a decade's worth of examples, we highlight how the design and synthesis of such libraries have been enabled by modern synthetic chemistry, and we illustrate the impact of the resultant chemical probes and drug leads in a wide range of diseases.
Figures

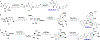




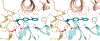
References
-
- Plenge RM Disciplined approach to drug discovery and early development. Sci. Transl. Med 8, 349ps15 (2016). - PubMed
-
- Holohan C, Van Schaeybroeck S, Longley DB & Johnston PG Cancer drug resistance: an evolving paradigm. Nat. Rev. Cancer 13, 714–726 (2013). - PubMed
-
- Blair JMA, Webber MA, Baylay AJ, Ogbolu DO & Piddock LJV Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol 13, 42–51 (2015). - PubMed
-
- Koehn FE & Carter GT The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov 4, 206–220 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

