CEBPE-Mutant Specific Granule Deficiency Correlates With Aberrant Granule Organization and Substantial Proteome Alterations in Neutrophils
- PMID: 29651288
- PMCID: PMC5884887
- DOI: 10.3389/fimmu.2018.00588
CEBPE-Mutant Specific Granule Deficiency Correlates With Aberrant Granule Organization and Substantial Proteome Alterations in Neutrophils
Abstract
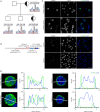
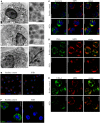
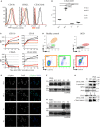
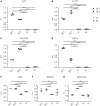
Specific granule deficiency (SGD) is a rare disorder characterized by abnormal neutrophils evidenced by reduced granules, absence of granule proteins, and atypical bilobed nuclei. Mutations in CCAAT/enhancer-binding protein-ε (CEBPE) are one molecular etiology of the disease. Although C/EBPε has been studied extensively, the impact of CEBPE mutations on neutrophil biology remains elusive. Here, we identified two SGD patients bearing a previously described heterozygous mutation (p.Val218Ala) in CEBPE. We took this rare opportunity to characterize SGD neutrophils in terms of granule distribution and protein content. Granules of patient neutrophils were clustered and polarized, suggesting that not only absence of specific granules but also defects affecting other granules contribute to the phenotype. Our analysis showed that remaining granules displayed mixed protein content and lacked several glycoepitopes. To further elucidate the impact of mutant CEBPE, we performed detailed proteomic analysis of SGD neutrophils. Beside an absence of several granule proteins in patient cells, we observed increased expression of members of the linker of nucleoskeleton and cytoskeleton complex (nesprin-2, vimentin, and lamin-B2), which control nuclear shape. This suggests that absence of these proteins in healthy individuals might be responsible for segmented shapes of neutrophilic nuclei. We further show that the heterozygous mutation p.Val218Ala in CEBPE causes SGD through prevention of nuclear localization of the protein product. In conclusion, we uncover that absence of nuclear C/EBPε impacts on spatiotemporal expression and subsequent distribution of several granule proteins and further on expression of proteins controlling nuclear shape.
Keywords: C/EBPε; granule organization; neutrophil granulocytes; primary immunodeficiency; specific granule deficiency.
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

