Total cardiovascular events analysis of the EXAMINE trial in patients with type 2 diabetes and recent acute coronary syndrome
- PMID: 29652078
- PMCID: PMC6489788
- DOI: 10.1002/clc.22960
Total cardiovascular events analysis of the EXAMINE trial in patients with type 2 diabetes and recent acute coronary syndrome
Abstract
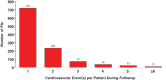
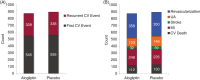
Alogliptin, a dipeptidyl peptidase-4 inhibitor, is approved for the treatment of patients with type 2 diabetes (T2DM). EXAMINE was a randomized controlled clinical trial designed to demonstrate the cardiovascular (CV) safety of alogliptin. In the trial, 5380 patients with established T2DM who had a recent acute coronary syndrome event (between 15 and 90 days) were randomized to treatment with either alogliptin or placebo. To better understand and describe the CV safety of alogliptin, we analyzed data from the EXAMINE trial to determine whether treatment with alogliptin affected recurrent and total CV events. Poisson regression analysis compared the total number of occurrences of CV death, MI, stroke, unstable angina, and coronary revascularization between all patients randomized to alogliptin vs placebo groups. Patients with recurrent CV events were older and more likely to have renal disease and history of heart failure. There were 1100 first CV events and an additional 666 recurrent events over a median of 18 months of follow-up. There were no significant differences with regard to total number of events in patients treated with alogliptin (n = 873) or placebo (n = 893; P = 0.52). Furthermore, there were no differences in the types of events seen in patients treated with alogliptin or placebo. Alogliptin did not increase the risk of either first or recurrent CV events when compared with placebo in patients with T2DM and recent acute coronary syndrome. These data support the CV safety of alogliptin in patients who are at increased risk of future CV events.
Keywords: Acute Coronary Syndrome; Dipeptidyl Peptidase-4 Inhibitors; Myocardial Infarction; Type 2 Diabetes.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
Dr. Cavender reports consulting fees from AstraZeneca, Chiesi, Merck, Sanofi‐Aventis, Boehringer Ingelheim, Janssen, and Novo Nordisk; he has received research funding (nonsalary) from Abbott Laboratories, AstraZeneca, Chiesi, GlaxoSmithKline, Merck, Novartis, and Takeda. Dr. White reports personal fees from Takeda USA, during the conduct of the study, and personal fees from AstraZeneca, Novartis, AbbVie, Sanofi‐Aventis, GlaxoSmithKline, and Pfizer Consumer Healthcare; he has received nonfinancial support from Roche Inc. and Wolters Kluwer, outside the submitted work. Dr Bergenstal has received research support, consulted, or has been on the scientific advisory board for Boehringer Ingelheim, Bristol‐Myers Squibb, AstraZeneca, Eli Lilly, Merck, Novo Nordisk, Roche, Sanofi, and Takeda; his employer, the nonprofit HealthPartners Institute, contracts for his services, and no personal income goes to Dr Bergenstal; also, he has inherited Merck stock. Dr. Zannad reports personal trial oversight committees and/or consulting fees from Amgen, AstraZeneca, Janssen, Bayer, Boehringer, Boston Scientific, CVRx, General Electric, Quantum Genomics, LivaNova, Mitsubishi, Novartis, Novo Nordisk, and Vifor Fresenius, and being the founder of CardioRenal and Cardiovascular Clinical Trialists. Dr. Heller has received personal fees from Takeda Development Center, Novo Nordisk, Eli Lilly, and Boeringher Ingelheim, and has served on speaker bureaus for Eli Lilly, Novo Nordisk, and AstraZeneca. Dr. Cushman has provided uncompensated consulting to Takeda and Novartis. Dr. Cannon reports research grants from Amgen, Arisaph, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Janssen, Merck, and Takeda. He reports consulting fees from Alnylam, Amgen, Amarin, Arisaph, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Eisai, GlaxoSmithKline, Kowa, LipimetiX, Merck, Pfizer, Regeneron, Sanofi, and Takeda. The authors declare no other potential conflicts of interest.
Figures
References
-
- White WB, Cannon CP, Heller SR, et al; EXAMINE Investigators . Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. - PubMed
-
- White WB, Bakris GL, Bergenstal RM, et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase‐4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J. 2011;162:620.e1–626.e1. - PubMed
-
- Zannad F, Cannon CP, Cushman WC, et al; EXAMINE Investigators . Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double‐blind trial. Lancet. 2015;385:2067–2076. - PubMed
-
- Hicks KA, Tcheng JE, Bozkurt B, et al. 2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards) [published correction appears in J Am Coll Cardiol 2015;66:982]. J Am Coll Cardiol. 2015;66:403–469. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

