Zero-Dimensional Cesium Lead Halides: History, Properties, and Challenges
- PMID: 29652149
- PMCID: PMC5937914
- DOI: 10.1021/acs.jpclett.8b00572
Zero-Dimensional Cesium Lead Halides: History, Properties, and Challenges
Abstract
Over the past decade, lead halide perovskites (LHPs) have emerged as new promising materials in the fields of photovoltaics and light emission due to their facile syntheses and exciting optical properties. The enthusiasm generated by LHPs has inspired research in perovskite-related materials, including the so-called "zero-dimensional cesium lead halides", which will be the focus of this Perspective. The structure of these materials is formed of disconnected lead halide octahedra that are stabilized by cesium ions. Their optical properties are dominated by optical transitions that are localized within the individual octahedra, hence the title "'zero-dimensional perovskites". Controversial results on their physical properties have recently been reported, and the true nature of their photoluminescence is still unclear. In this Perspective, we will take a close look at these materials, both as nanocrystals and as bulk crystals/thin films, discuss the contrasting opinions on their properties, propose potential applications, and provide an outlook on future experiments.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
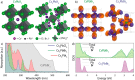


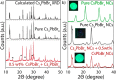

References
-
- Goldschmidt V. M. Die Gesetze Der Krystallochemie. Naturwissenschaften 1926, 14, 477–485. 10.1007/BF01507527. - DOI
-
- Wells H. L. Über Die Cäsium- Und Kalium-Bleihalogenide. Z. anorg. allg. Chem. 1893, 3, 195–210. 10.1002/zaac.18930030124. - DOI
-
- Nikl M.; Mihokova E.; Nitsch K. Photoluminescence & Decay Kinetics of Cs4PbCl6 Single Crystals. Solid State Commun. 1992, 84, 1089–1092. 10.1016/0038-1098(92)90691-2. - DOI
-
- Nikl M.; et al. Quantum Size Effect in the Excitonic Luminescence of CsPbX3-Like Quantum Dots in CsX (X = Cl, Br) Single Crystal Host. J. Lumin. 1997, 72-74, 377–379. 10.1016/S0022-2313(96)00341-9. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

