n-3 Fatty Acid Supplementation for the Treatment of Dry Eye Disease
- PMID: 29652551
- PMCID: PMC5952353
- DOI: 10.1056/NEJMoa1709691
n-3 Fatty Acid Supplementation for the Treatment of Dry Eye Disease
Abstract
Background: Dry eye disease is a common chronic condition that is characterized by ocular discomfort and visual disturbances that decrease quality of life. Many clinicians recommend the use of supplements of n-3 fatty acids (often called omega-3 fatty acids) to relieve symptoms.
Methods: In a multicenter, double-blind clinical trial, we randomly assigned patients with moderate-to-severe dry eye disease to receive a daily oral dose of 3000 mg of fish-derived n-3 eicosapentaenoic and docosahexaenoic acids (active supplement group) or an olive oil placebo (placebo group). The primary outcome was the mean change from baseline in the score on the Ocular Surface Disease Index (OSDI; scores range from 0 to 100, with higher scores indicating greater symptom severity), which was based on the mean of scores obtained at 6 and 12 months. Secondary outcomes included mean changes per eye in the conjunctival staining score (ranging from 0 to 6) and the corneal staining score (ranging from 0 to 15), with higher scores indicating more severe damage to the ocular surface, as well as mean changes in the tear break-up time (seconds between a blink and gaps in the tear film) and the result on Schirmer's test (length of wetting of paper strips placed on the lower eyelid), with lower values indicating more severe signs.
Results: A total of 349 patients were assigned to the active supplement group and 186 to the placebo group; the primary analysis included 329 and 170 patients, respectively. The mean change in the OSDI score was not significantly different between the active supplement group and the placebo group (-13.9 points and -12.5 points, respectively; mean difference in change after imputation of missing data, -1.9 points; 95% confidence interval [CI], -5.0 to 1.1; P=0.21). This result was consistent across prespecified subgroups. There were no significant differences between the active supplement group and the placebo group in mean changes from baseline in the conjunctival staining score (mean difference in change, 0.0 points; 95% CI, -0.2 to 0.1), corneal staining score (0.1 point; 95% CI, -0.2 to 0.4), tear break-up time (0.2 seconds; 95% CI, -0.1 to 0.5), and result on Schirmer's test (0.0 mm; 95% CI, -0.8 to 0.9). At 12 months, the rate of adherence to treatment in the active supplement group was 85.2%, according to the level of n-3 fatty acids in red cells. Rates of adverse events were similar in the two trial groups.
Conclusions: Among patients with dry eye disease, those who were randomly assigned to receive supplements containing 3000 mg of n-3 fatty acids for 12 months did not have significantly better outcomes than those who were assigned to receive placebo. (Funded by the National Eye Institute, National Institutes of Health; DREAM ClinicalTrials.gov number, NCT02128763 .).
Figures
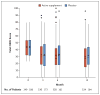
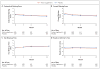
Comment in
-
n-3 Fatty Acid Supplementation and Dry Eye Disease.N Engl J Med. 2018 Aug 16;379(7):690-691. doi: 10.1056/NEJMc1807693. N Engl J Med. 2018. PMID: 30110584 No abstract available.
-
n-3 Fatty Acid Supplementation and Dry Eye Disease.N Engl J Med. 2018 Aug 16;379(7):691. doi: 10.1056/NEJMc1807693. N Engl J Med. 2018. PMID: 30124262 No abstract available.
-
Why DREAM should make you think twice about recommending Omega-3 supplements.Ocul Surf. 2019 Oct;17(4):617-618. doi: 10.1016/j.jtos.2019.08.003. Epub 2019 Aug 12. Ocul Surf. 2019. PMID: 31415816 No abstract available.
References
-
- Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–83. - PubMed
-
- Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15:334–65. - PubMed
-
- Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea. 2011;30:379–87. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
