Hypoxia Supports Epicardial Cell Differentiation in Vascular Smooth Muscle Cells through the Activation of the TGFβ Pathway
- PMID: 29652803
- PMCID: PMC6023394
- DOI: 10.3390/jcdd5020019
Hypoxia Supports Epicardial Cell Differentiation in Vascular Smooth Muscle Cells through the Activation of the TGFβ Pathway
Abstract
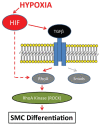
Epicardium-derived cells (EPDCs) are an important pool of multipotent cardiovascular progenitor cells. Through epithelial-to-mesenchymal-transition (EMT), EPDCs invade the subepicardium and myocardium and further differentiate into several cell types required for coronary vessel formation. We previously showed that epicardial hypoxia inducible factor (HIF) signaling mediates the invasion of vascular precursor cells critical for patterning the coronary vasculature. Here, we examine the regulatory role of hypoxia (1% oxygen) on EPDC differentiation into vascular smooth muscle cells (VSMCs).
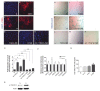
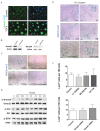
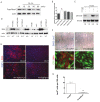
Results: Hypoxia stimulates EMT and enhances expression of several VSMC markers in mouse epicardial cell cultures. This stimulation is specifically blocked by inhibiting transforming growth factor-beta (TGFβ) receptor I. Further analyses indicated that hypoxia increases the expression level of TGFβ-1 ligand and phosphorylation of TGFβ receptor II, suggesting an indispensable role of the TGFβ pathway in hypoxia-stimulated VSMC differentiation. We further demonstrate that the non-canonical RhoA/Rho kinase (ROCK) pathway acts as the main downstream effector of TGFβ to modulate hypoxia’s effect on VSMC differentiation.
Conclusion: Our results reveal a novel role of epicardial HIF in mediating coronary vasculogenesis by promoting their differentiation into VSMCs through noncanonical TGFβ signaling. These data elucidate that patterning of the coronary vasculature is influenced by epicardial hypoxic signals.
Keywords: RhoA/ROCK; TGFβ; coronary vasculature; epicardial cell; epithelial-to-mesenchymal-transition; hypoxia; hypoxia inducible factor; transforming growth factor beta; vascular smooth muscle cells.
Conflict of interest statement
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript and in the decision to publish the results.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

