Excessive Extracellular ATP Desensitizes P2Y2 and P2X4 ATP Receptors Provoking Surfactant Impairment Ending in Ventilation-Induced Lung Injury
- PMID: 29652806
- PMCID: PMC5979391
- DOI: 10.3390/ijms19041185
Excessive Extracellular ATP Desensitizes P2Y2 and P2X4 ATP Receptors Provoking Surfactant Impairment Ending in Ventilation-Induced Lung Injury
Abstract
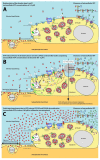
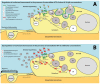
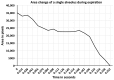
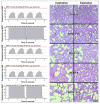
Stretching the alveolar epithelial type I (AT I) cells controls the intercellular signaling for the exocytosis of surfactant by the AT II cells through the extracellular release of adenosine triphosphate (ATP) (purinergic signaling). Extracellular ATP is cleared by extracellular ATPases, maintaining its homeostasis and enabling the lung to adapt the exocytosis of surfactant to the demand. Vigorous deformation of the AT I cells by high mechanical power ventilation causes a massive release of extracellular ATP beyond the clearance capacity of the extracellular ATPases. When extracellular ATP reaches levels >100 μM, the ATP receptors of the AT II cells become desensitized and surfactant impairment is initiated. The resulting alteration in viscoelastic properties and in alveolar opening and collapse time-constants leads to alveolar collapse and the redistribution of inspired air from the alveoli to the alveolar ducts, which become pathologically dilated. The collapsed alveoli connected to these dilated alveolar ducts are subject to a massive strain, exacerbating the ATP release. After reaching concentrations >300 μM extracellular ATP acts as a danger-associated molecular pattern, causing capillary leakage, alveolar space edema, and further deactivation of surfactant by serum proteins. Decreasing the tidal volume to 6 mL/kg or less at this stage cannot prevent further lung injury.
Keywords: P2X receptors; P2Y receptors; extracellular ATP; innate immunity; purinergic signaling; surfactant dysfunction; ventilation-induced lung injury.
Conflict of interest statement
Djo Hasan: The author reports to have received an honorarium to a lecture from Demcon-Macawi; Michaela Kollisch-Singule: The author reports to have received travel reimbursement at events sponsored by Dräger Medical; Gary F. Nieman.: The author reports to have received grants from the NIH, a grant from Dräger Medical, a grant from the CDRMP (DoD), travel and honorarium to lecture from Dräger Medical; Joshua Satalin, Philip van der Zee, Paul Blankman, Atsuko Shono, Peter Somhorst, Corstiaan den Uil, Han Meeder and Toru Kotani: The authors declare no conflicts of interest.
Figures
References
-
- Lohmann K. Über die Pyrophosphatfraktion im Muskel. Naturwissenschaften. 1929;17:624–625. doi: 10.1007/BF01506215. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

