Sympathetic Nerve Hyperactivity in the Spleen: Causal for Nonpathogenic-Driven Chronic Immune-Mediated Inflammatory Diseases (IMIDs)?
- PMID: 29652832
- PMCID: PMC5979464
- DOI: 10.3390/ijms19041188
Sympathetic Nerve Hyperactivity in the Spleen: Causal for Nonpathogenic-Driven Chronic Immune-Mediated Inflammatory Diseases (IMIDs)?
Abstract
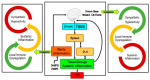
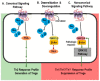
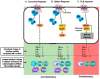
Immune-Mediated Inflammatory Diseases (IMIDs) is a descriptive term coined for an eclectic group of diseases or conditions that share common inflammatory pathways, and for which there is no definitive etiology. IMIDs affect the elderly most severely, with many older individuals having two or more IMIDs. These diseases include, but are not limited to, type-1 diabetes, obesity, hypertension, chronic pulmonary disease, coronary heart disease, inflammatory bowel disease, and autoimmunity, such as rheumatoid arthritis (RA), Sjőgren's syndrome, systemic lupus erythematosus, psoriasis, psoriatic arthritis, and multiple sclerosis. These diseases are ostensibly unrelated mechanistically, but increase in frequency with age and share chronic systemic inflammation, implicating major roles for the spleen. Chronic systemic and regional inflammation underlies the disease manifestations of IMIDs. Regional inflammation and immune dysfunction promotes targeted end organ tissue damage, whereas systemic inflammation increases morbidity and mortality by affecting multiple organ systems. Chronic inflammation and skewed dysregulated cell-mediated immune responses drive many of these age-related medical disorders. IMIDs are commonly autoimmune-mediated or suspected to be autoimmune diseases. Another shared feature is dysregulation of the autonomic nervous system and hypothalamic pituitary adrenal (HPA) axis. Here, we focus on dysautonomia. In many IMIDs, dysautonomia manifests as an imbalance in activity/reactivity of the sympathetic and parasympathetic divisions of the autonomic nervous system (ANS). These major autonomic pathways are essential for allostasis of the immune system, and regulating inflammatory processes and innate and adaptive immunity. Pathology in ANS is a hallmark and causal feature of all IMIDs. Chronic systemic inflammation comorbid with stress pathway dysregulation implicate neural-immune cross-talk in the etiology and pathophysiology of IMIDs. Using a rodent model of inflammatory arthritis as an IMID model, we report disease-specific maladaptive changes in β₂-adrenergic receptor (AR) signaling from protein kinase A (PKA) to mitogen activated protein kinase (MAPK) pathways in the spleen. Beta₂-AR signal "shutdown" in the spleen and switching from PKA to G-coupled protein receptor kinase (GRK) pathways in lymph node cells drives inflammation and disease advancement. Based on these findings and the existing literature in other IMIDs, we present and discuss relevant literature that support the hypothesis that unresolvable immune stimulation from chronic inflammation leads to a maladaptive disease-inducing and perpetuating sympathetic response in an attempt to maintain allostasis. Since the role of sympathetic dysfunction in IMIDs is best studied in RA and rodent models of RA, this IMID is the primary one used to evaluate data relevant to our hypothesis. Here, we review the relevant literature and discuss sympathetic dysfunction as a significant contributor to the pathophysiology of IMIDs, and then discuss a novel target for treatment. Based on our findings in inflammatory arthritis and our understanding of common inflammatory process that are used by the immune system across all IMIDs, novel strategies to restore SNS homeostasis are expected to provide safe, cost-effective approaches to treat IMIDs, lower comorbidities, and increase longevity.
Keywords: G protein receptor kinase; adrenergic receptor signaling; immune-mediated inflammatory diseases; inflammatory reflex; mitogen-activated protein kinase; neural-immune; protein kinase A; rheumatoid arthritis; sympathetic nervous system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

