Glucose sensor-augmented continuous subcutaneous insulin infusion in patients with diabetic gastroparesis: An open-label pilot prospective study
- PMID: 29652893
- PMCID: PMC5898706
- DOI: 10.1371/journal.pone.0194759
Glucose sensor-augmented continuous subcutaneous insulin infusion in patients with diabetic gastroparesis: An open-label pilot prospective study
Abstract
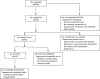
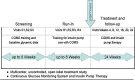
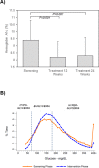
Erratic blood glucose levels can be a cause and consequence of delayed gastric emptying in patients with diabetes. It is unknown if better glycemic control increases risks of hypoglycemia or improves hemoglobin A1c levels and gastrointestinal symptoms in diabetic gastroparesis. This study investigated the safety and potential efficacy of continuous subcutaneous insulin infusion (CSII) and continuous glucose monitoring (CGM) in poorly controlled diabetes with gastroparesis. Forty-five type 1 or 2 patients with diabetes and gastroparesis and hemoglobin A1c >8% from the NIDDK Gastroparesis Consortium enrolled in a 24 week open-label pilot prospective study of CSII plus CGM. The primary safety outcome was combined numbers of mild, moderate, and severe hypoglycemic events at screening and 24 weeks treatment. Secondary outcomes included glycemic excursions on CGM, hemoglobin A1c, gastroparesis symptoms, quality-of-life, and liquid meal tolerance. Combined mild, moderate, and severe hypoglycemic events occurred similarly during the screening/run-in (1.9/week) versus treatment (2.2/week) phases with a relative risk of 1.18 (95% CI 0.85-1.64, P = 0.33). CGM time in hypoglycemia (<70 mg/dL) decreased from 3.9% to 1.8% (P<0.0001), time in euglycemia (70-180 mg/dL) increased from 44.0% to 52.0% (P = 0.02), time in severe hyperglycemia (>300 mg/dL) decreased from 14.2% to 7.0% (P = 0.005), and hemoglobin A1c decreased from 9.4±1.4% to 8.3±1.3% (P = 0.001) on CSII plus CGM. Symptom scores decreased from 29.3±7.1 to 21.9±10.2 with lower nausea/vomiting, fullness/early satiety, and bloating/distention scores (P≤0.001). Quality-of-life scores improved from 2.4±1.1 to 3.1±1.1 (P<0.0001) and volumes of liquid nutrient meals tolerated increased from 420±258 to 487±312 mL (P = 0.05) at 24 weeks. In conclusion, CSII plus CGM appeared to be safe with minimal risks of hypoglycemic events and associated improvements in glycemic control, gastroparesis symptoms, quality-of-life, and meal tolerance in patients with poorly controlled diabetes and gastroparesis. This study supports the safety, feasibility, and potential benefits of improving glycemic control in diabetic gastroparesis.
Conflict of interest statement
Figures
References
-
- Camilleri M. Gastrointestinal problems in diabetes. Endocrinol Metab Clin North Am. 1996; 25: 361–378. - PubMed
-
- Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med. 2007; 356: 820–829. doi: 10.1056/NEJMcp062614 - DOI - PubMed
-
- Uppalapati SS, Ramzan Z, Fisher RS, Parkman HP. Factors contributing to hospitalization for gastroparesis exacerbations. Dig Dis Sci. 2009; 54: 2404–2409. doi: 10.1007/s10620-009-0975-1 - DOI - PubMed
-
- Pasricha PJ, Yates KP, Nguyen L, Clarke J, Abell TL, Farrugia G, et al. Outcomes and factors associated with reduced symptoms in patients with gastroparesis. Gastroenterology. 2015; 149: 1762–1774. doi: 10.1053/j.gastro.2015.08.008 - DOI - PMC - PubMed
-
- Bytzer P, Talley NJ, Hammer J, Young LJ, Jones MP, Horowitz M. GI symptoms in diabetes mellitus are associated with both poor glycemic control and diabetic complications. Am J Gastroenterol. 2002; 97: 604–611. doi: 10.1111/j.1572-0241.2002.05537.x - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 DK074008/DK/NIDDK NIH HHS/United States
- U01DK073983 /NH/NIH HHS/United States
- P30 DK092926/DK/NIDDK NIH HHS/United States
- U01DK073975 /NH/NIH HHS/United States
- U01 DK074007/DK/NIDDK NIH HHS/United States
- U01DK073974 /NH/NIH HHS/United States
- U01 DK073985/DK/NIDDK NIH HHS/United States
- U01 DK073975/DK/NIDDK NIH HHS/United States
- U01 DK074035/DK/NIDDK NIH HHS/United States
- U01DK073985 /NH/NIH HHS/United States
- U01DK074007 /NH/NIH HHS/United States
- U01DK074008 /NH/NIH HHS/United States
- U01DK074035 /NH/NIH HHS/United States
- U24 DK074008/DK/NIDDK NIH HHS/United States
- U01 DK073983/DK/NIDDK NIH HHS/United States
- U01 DK112193/DK/NIDDK NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- U01 DK073974/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

