Pluripotent stem cell-based therapy for Parkinson's disease: Current status and future prospects
- PMID: 29653250
- PMCID: PMC6077089
- DOI: 10.1016/j.pneurobio.2018.04.005
Pluripotent stem cell-based therapy for Parkinson's disease: Current status and future prospects
Abstract
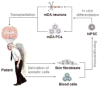
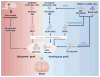
Parkinson's disease (PD) is one of the most common neurodegenerative disorders, which affects about 0.3% of the general population. As the population in the developed world ages, this creates an escalating burden on society both in economic terms and in quality of life for these patients and for the families that support them. Although currently available pharmacological or surgical treatments may significantly improve the quality of life of many patients with PD, these are symptomatic treatments that do not slow or stop the progressive course of the disease. Because motor impairments in PD largely result from loss of midbrain dopamine neurons in the substantia nigra pars compacta, PD has long been considered to be one of the most promising target diseases for cell-based therapy. Indeed, numerous clinical and preclinical studies using fetal cell transplantation have provided proof of concept that cell replacement therapy may be a viable therapeutic approach for PD. However, the use of human fetal cells as a standardized therapeutic regimen has been fraught with fundamental ethical, practical, and clinical issues, prompting scientists to explore alternative cell sources. Based on groundbreaking establishments of human embryonic stem cells and induced pluripotent stem cells, these human pluripotent stem cells have been the subject of extensive research, leading to tremendous advancement in our understanding of these novel classes of stem cells and promising great potential for regenerative medicine. In this review, we discuss the prospects and challenges of human pluripotent stem cell-based cell therapy for PD.
Keywords: Induced pluripotent stem cell; Midbrain dopamine neuron; Parkinson’s disease; Personalized cell therapy; Pluripotent stem cell-based therapy; Transplantation.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures


References
-
- Ahlskog JE, Kelly PJ, van Heerden JA, Stoddard SL, Tyce GM, Windebank AJ, Bailey PA, Bell GN, Blexrud MD, Carmichael SW. Adrenal medullary transplantation into the brain for treatment of Parkinson’s disease: clinical outcome and neurochemical studies. Mayo Clin Proc. 1990;65:305–328. - PubMed
-
- Allan LE, Petit GH, Brundin P. Cell transplantation in Parkinson’s disease: problems and perspectives. Curr Opin Neurol. 2010;23:426–432. - PubMed
-
- Amano T, Papanikolaou T, Sung LY, Lennington J, Conover J, Yang X. Nuclear transfer embryonic stem cells provide an in vitro culture model for Parkinson’s disease. Cloning Stem Cells. 2009;11:77–88. - PubMed
-
- Amir H, Touboul T, Sabatini K, Chhabra D, Garitaonandia I, Loring JF, Morey R, Laurent LC. Spontaneous Single-Copy Loss of TP53 in Human Embryonic Stem Cells Markedly Increases Cell Proliferation and Survival. Stem Cells. 2017;35:872–885. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

