Autophagy inhibition attenuates hyperoxaluria-induced renal tubular oxidative injury and calcium oxalate crystal depositions in the rat kidney
- PMID: 29653411
- PMCID: PMC5953241
- DOI: 10.1016/j.redox.2018.03.019
Autophagy inhibition attenuates hyperoxaluria-induced renal tubular oxidative injury and calcium oxalate crystal depositions in the rat kidney
Abstract
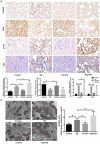
Hyperoxaluria-induced oxidative injury of renal tubular epithelial cell is a casual and essential factor in kidney calcium oxalate (CaOx) stone formation. Autophagy has been shown to be critical for the regulation of oxidative stress-induced renal tubular injury; however, little is known about its role in kidney CaOx stone formation. In the present study, we found that the autophagy antagonist chloroquine could significantly attenuate oxalate-induced autophagy activation, oxidative injury and mitochondrial damage of renal tubular cells in vitro and in vivo, as well as hyperoxaluria-induced CaOx crystals depositions in rat kidney, whereas the autophagy agonist rapamycin exerted contrasting effects. In addition, oxalate-induced p38 phosphorylation was significantly attenuated by chloroquine pretreatment but was markedly enhanced by rapamycin pretreatment, whereas the protective effect of chloroquine on rat renal tubular cell oxidative injury was partly reversed by a p38 protein kinase activator anisomycin. Furthermore, the knockdown of Beclin1 represented similar effects to chloroquine on oxalate-induced cell oxidative injury and p38 phosphorylation in vitro. Taken together, our results revealed that autophagy inhibition could attenuate oxalate-induced oxidative injury of renal tubular cell and CaOx crystal depositions in the rat kidney via, at least in part, inhibiting the activation of p38 signaling pathway, thus representing a novel role of autophagy in the regulation of oxalate-induced renal oxidative injury and CaOx crystal depositions for the first time.
Keywords: Autophagy; Calcium oxalate stone; Oxalate; Oxidative injury; p38.
Copyright © 2018. Published by Elsevier B.V.
Figures






References
-
- Zeng G., Mai Z., Xia S., Wang Z., Zhang K., Wang L., Long Y., Ma J., Li Y., Wan S.P., Wu W., Liu Y., Cui Z., Zhao Z., Qin J., Zeng T., Liu Y., Duan X., Mai X., Yang Z., Kong Z., Zhang T., Cai C., Shao Y., Yue Z., Li S., Ding J., Tang S., Ye Z. Prevalence of kidney stones in China: an ultrasonography based cross-sectional study. BJU Int. 2017;120:109–116. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

