A Novel Amino Lipid Series for mRNA Delivery: Improved Endosomal Escape and Sustained Pharmacology and Safety in Non-human Primates
- PMID: 29653760
- PMCID: PMC5986714
- DOI: 10.1016/j.ymthe.2018.03.010
A Novel Amino Lipid Series for mRNA Delivery: Improved Endosomal Escape and Sustained Pharmacology and Safety in Non-human Primates
Abstract
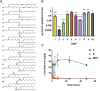
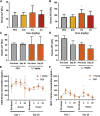
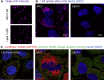
The success of mRNA-based therapies depends on the availability of a safe and efficient delivery vehicle. Lipid nanoparticles have been identified as a viable option. However, there are concerns whether an acceptable tolerability profile for chronic dosing can be achieved. The efficiency and tolerability of lipid nanoparticles has been attributed to the amino lipid. Therefore, we developed a new series of amino lipids that address this concern. Clear structure-activity relationships were developed that resulted in a new amino lipid that affords efficient mRNA delivery in rodent and primate models with optimal pharmacokinetics. A 1-month toxicology evaluation in rat and non-human primate demonstrated no adverse events with the new lipid nanoparticle system. Mechanistic studies demonstrate that the improved efficiency can be attributed to increased endosomal escape. This effort has resulted in the first example of the ability to safely repeat dose mRNA-containing lipid nanoparticles in non-human primate at therapeutically relevant levels.
Keywords: delivery; lipid nanoparticle; mRNA; mRNA therapeutics; safe chronic dosing; tolerability.
Copyright © 2018 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics—developing a new class of drugs. Nat. Rev. Drug Discov. 2014;13:759–780. - PubMed
-
- Hajj K.A., Whitehead K.A. Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2017;2:17056.
-
- Stanton M.G., Murphy-Benenato K.E. Messenger RNA as a novel therapeutic approach. In: Garner A., editor. Volume 27. Springer; 2017. pp. 237–253. (Topics in Medicinal Chemistry: RNA Therapeutics).
-
- Coelho T., Adams D., Silva A., Lozeron P., Hawkins P.N., Mant T., Perez J., Chiesa J., Warrington S., Tranter E. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N. Engl. J. Med. 2013;369:819–829. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

