Effect of ready-to-use supplementary food on mortality in severely immunocompromised HIV-infected individuals in Africa initiating antiretroviral therapy (REALITY): an open-label, parallel-group, randomised controlled trial
- PMID: 29653915
- PMCID: PMC5932190
- DOI: 10.1016/S2352-3018(18)30038-9
Effect of ready-to-use supplementary food on mortality in severely immunocompromised HIV-infected individuals in Africa initiating antiretroviral therapy (REALITY): an open-label, parallel-group, randomised controlled trial
Erratum in
-
Corrections.Lancet HIV. 2018 Jul;5(7):e340. doi: 10.1016/S2352-3018(18)30113-9. Epub 2018 May 29. Lancet HIV. 2018. PMID: 29858037 Free PMC article. No abstract available.
Abstract
Background: In sub-Saharan Africa, severely immunocompromised HIV-infected individuals have a high risk of mortality during the first few months after starting antiretroviral therapy (ART). We hypothesise that universally providing ready-to-use supplementary food (RUSF) would increase early weight gain, thereby reducing early mortality compared with current guidelines recommending ready-to-use therapeutic food (RUTF) for severely malnourished individuals only.
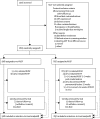
Methods: We did a 2 × 2 × 2 factorial, open-label, parallel-group trial at inpatient and outpatient facilities in eight urban or periurban regional hospitals in Kenya, Malawi, Uganda, and Zimbabwe. Eligible participants were ART-naive adults and children aged at least 5 years with confirmed HIV infection and a CD4 cell count of fewer than 100 cells per μL, who were initiating ART at the facilities. We randomly assigned participants (1:1) to initiate ART either with (RUSF) or without (no-RUSF) 12 weeks' of peanut-based RUSF containing 1000 kcal per day and micronutrients, given as two 92 g packets per day for adults and one packet (500 kcal per day) for children aged 5-12 years, regardless of nutritional status. In both groups, individuals received supplementation with RUTF only when severely malnourished (ie, body-mass index [BMI] <16-18 kg/m2 or BMI-for-age Z scores <-3 for children). We did the randomisation with computer-generated, sequentially numbered tables with different block sizes incorporated within an online database. Randomisation was stratified by centre, age, and two other factorial randomisations, to 12 week adjunctive raltegravir and enhanced anti-infection prophylaxis (reported elsewhere). Clinic visits were scheduled at weeks 2, 4, 8, 12, 18, 24, 36, and 48, and included nurse assessment of vital status and symptoms and dispensing of all medication including ART and RUSF. The primary outcome was mortality at week 24, analysed by intention to treat. Secondary outcomes included absolute changes in weight, BMI, and mid-upper-arm circumference (MUAC). Safety was analysed in all randomly assigned participants. Follow-up was 48 weeks. This trial is registered with ClinicalTrials.gov (NCT01825031) and the ISRCTN registry (43622374).
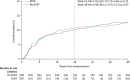
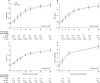
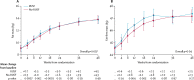
Findings: Between June 18, 2013, and April 10, 2015, we randomly assigned 1805 participants to treatment: 897 to RUSF and 908 to no-RUSF. 56 (3%) were lost-to-follow-up. 96 (10·9%, 95% CI 9·0-13·1) participants allocated to RUSF and 92 (10·3%, 8·5-12·5) to no-RUSF died within 24 weeks (hazard ratio 1·05, 95% CI 0·79-1·40; log-rank p=0·75), with no evidence of interaction with the other randomisations (both p>0·7). Through 48 weeks, adults and adolescents aged 13 years and older in the RUSF group had significantly greater gains in weight, BMI, and MUAC than the no-RUSF group (p=0·004, 0·004, and 0·03, respectively). The most common type of serious adverse event was specific infections, occurring in 90 (10%) of 897 participants assigned RUSF and 87 (10%) of 908 assigned no-RUSF. By week 48, 205 participants had serious adverse events in both groups (p=0·81), and 181 had grade 4 adverse events in the RUSF group compared with 172 in the non-RUSF group (p=0·45).
Interpretation: In severely immunocompromised HIV-infected individuals, providing RUSF universally at ART initiation, compared with providing RUTF to severely malnourished individuals only, improved short-term weight gain but not mortality. A change in policy to provide nutritional supplementation to all severely immunocompromised HIV-infected individuals starting ART is therefore not warranted at present.
Funding: Joint Global Health Trials Scheme (UK Medical Research Council, UK Department for International Development, and Wellcome Trust).
Copyright © 2018 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY NC ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Nutritional support to reduce mortality in patients with HIV?Lancet HIV. 2018 May;5(5):e202-e204. doi: 10.1016/S2352-3018(18)30047-X. Epub 2018 Apr 10. Lancet HIV. 2018. PMID: 29653916 No abstract available.
References
-
- WHO . second edn. World health Organization; Geneva: 2016. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach.http://www.who.int/hiv/pub/arv/arv-2016/en/ (accessed Jan 28, 2018). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- MC_UU_12023/17/MRC_/Medical Research Council/United Kingdom
- MC_EX_G1100693/MRC_/Medical Research Council/United Kingdom
- MC_UU_12023/26/MRC_/Medical Research Council/United Kingdom
- MR/M007367/1/MRC_/Medical Research Council/United Kingdom
- MC_EX_UU_G1100693/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

