Remote Atmospheric Pressure Infrared Matrix-Assisted Laser Desorption-Ionization Mass Spectrometry (Remote IR-MALDI MS) of Proteins
- PMID: 29653959
- PMCID: PMC6072536
- DOI: 10.1074/mcp.TIR117.000582
Remote Atmospheric Pressure Infrared Matrix-Assisted Laser Desorption-Ionization Mass Spectrometry (Remote IR-MALDI MS) of Proteins
Abstract
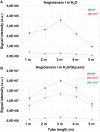
Remote Infrared Matrix-Assisted Laser Desorption/Ionization (Remote IR-MALDI) system using tissue endogenous water as matrix was shown to enable in vivo real-time mass spectrometry analysis with minimal invasiveness. Initially the system was used to detect metabolites and lipids. Here, we demonstrate its capability to detect and analyze peptides and proteins. Very interestingly, the corresponding mass spectra show ESI-like charge state distribution, opening many applications for structural elucidation to be performed in real-time by Top-Down strategy. The charge states show no dependence toward laser wavelength or length of the transfer tube. Indeed, remote analysis can be performed 5 m away from the mass spectrometer without modification of spectra. On the contrary, addition of glycerol to water shift the charge state distributions toward even higher charge states. The desorption/ionization process is very soft, allowing to maintain protein conformation as in ESI. Observation of proteins and similar spectral features on tissue, when protein standards are deposited on raw tissue pieces, could potentially open the way to their direct analysis from biological samples. This also brings interesting features that could contribute to the understanding of IR MALDI ionization mechanism.
Keywords: Biophysical methods; Mass Spectrometry; Multiply Charged Ions; Protein Conformation; Protein Identification; Real-Time Analysis; Remote IR MALDI; SpiderMass; Tandem Mass Spectrometry; Top-Down.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures








References
-
- Huang M. Z., Cheng S. C., Cho Y. T., and Shiea J. (2011) Ambient ionization mass spectrometry: a tutorial. Anal. Chim. Acta 702, 1–15 - PubMed
-
- Harris G. A., Galhena A. S., and Fernandez F. M. (2011) Ambient sampling/ionization mass spectrometry: applications and current trends. Anal. Chem. 83, 4508–4538 - PubMed
-
- Alberici R. M., Simas R. C., Sanvido G. B., Romão W., Lalli P. M., Benassi M., Cunha I. B., and Eberlin M. N. (2010) Ambient mass spectrometry: bringing MS into the “real world”. Anal. Bioanal. Chem. 398, 265–294 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

