Endoplasmic reticulum stress activates SRC, relocating chaperones to the cell surface where GRP78/CD109 blocks TGF-β signaling
- PMID: 29654145
- PMCID: PMC5939063
- DOI: 10.1073/pnas.1714866115
Endoplasmic reticulum stress activates SRC, relocating chaperones to the cell surface where GRP78/CD109 blocks TGF-β signaling
Abstract
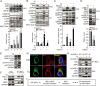
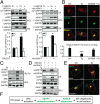
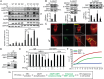
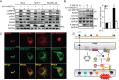
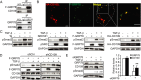
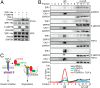
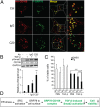
The discovery that endoplasmic reticulum (ER) luminal chaperones such as GRP78/BiP can escape to the cell surface upon ER stress where they regulate cell signaling, proliferation, apoptosis, and immunity represents a paradigm shift. Toward deciphering the mechanisms, we report here that, upon ER stress, IRE1α binds to and triggers tyrosine kinase SRC activation, leading to ASAP1 phosphorylation and Golgi accumulation of ASAP1 and Arf1-GTP, resulting in KDEL receptor dispersion from the Golgi and suppression of retrograde transport. At the cell surface, GRP78 binds to and acts in concert with a glycosylphosphatidylinositol-anchored protein, CD109, in blocking TGF-β signaling by promoting the routing of the TGF-β receptor to the caveolae, thereby disrupting its binding to and activation of Smad2. Collectively, we uncover a SRC-mediated signaling cascade that leads to the relocalization of ER chaperones to the cell surface and a mechanism whereby GRP78 counteracts the tumor-suppressor effect of TGF-β.
Keywords: GRP78; SRC protein kinase; TGF-β signaling; endoplasmic reticulum stress; retrograde transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Src phosphorylates Tyr284 in TGF-beta type II receptor and regulates TGF-beta stimulation of p38 MAPK during breast cancer cell proliferation and invasion.Cancer Res. 2007 Apr 15;67(8):3752-8. doi: 10.1158/0008-5472.CAN-06-3851. Cancer Res. 2007. PMID: 17440088
-
CD109-mediated degradation of TGF-β receptors and inhibition of TGF-β responses involve regulation of SMAD7 and Smurf2 localization and function.J Cell Biochem. 2012 Jan;113(1):238-46. doi: 10.1002/jcb.23349. J Cell Biochem. 2012. PMID: 21898545
-
GRP78 and Cripto form a complex at the cell surface and collaborate to inhibit transforming growth factor beta signaling and enhance cell growth.Mol Cell Biol. 2008 Jan;28(2):666-77. doi: 10.1128/MCB.01716-07. Epub 2007 Nov 8. Mol Cell Biol. 2008. PMID: 17991893 Free PMC article.
-
Cripto/GRP78 modulation of the TGF-β pathway in development and oncogenesis.FEBS Lett. 2012 Jul 4;586(14):1836-45. doi: 10.1016/j.febslet.2012.01.051. Epub 2012 Feb 1. FEBS Lett. 2012. PMID: 22306319 Free PMC article. Review.
-
The multifaceted role of the embryonic gene Cripto-1 in cancer, stem cells and epithelial-mesenchymal transition.Semin Cancer Biol. 2014 Dec;29:51-8. doi: 10.1016/j.semcancer.2014.08.003. Epub 2014 Aug 19. Semin Cancer Biol. 2014. PMID: 25153355 Free PMC article. Review.
Cited by
-
Dysregulation of the miR-30a/BiP axis by cigarette smoking accelerates oral cancer progression.Cancer Cell Int. 2021 Oct 30;21(1):578. doi: 10.1186/s12935-021-02276-1. Cancer Cell Int. 2021. PMID: 34717640 Free PMC article.
-
GENT2: an updated gene expression database for normal and tumor tissues.BMC Med Genomics. 2019 Jul 11;12(Suppl 5):101. doi: 10.1186/s12920-019-0514-7. BMC Med Genomics. 2019. PMID: 31296229 Free PMC article.
-
Prognostic Significance of CD109 Expression in Patients with Ovarian Epithelial Cancer.J Pathol Transl Med. 2019 Jul;53(4):244-252. doi: 10.4132/jptm.2019.04.16. Epub 2019 May 2. J Pathol Transl Med. 2019. PMID: 31316041 Free PMC article.
-
The chaperone GRP78 is a host auxiliary factor for SARS-CoV-2 and GRP78 depleting antibody blocks viral entry and infection.J Biol Chem. 2021 Jan-Jun;296:100759. doi: 10.1016/j.jbc.2021.100759. Epub 2021 May 7. J Biol Chem. 2021. PMID: 33965375 Free PMC article.
-
Baseline Values of Circulating IL-6 and TGF-β Might Identify Patients with HNSCC Who Do Not Benefit from Nivolumab Treatment.Cancers (Basel). 2023 Nov 2;15(21):5257. doi: 10.3390/cancers15215257. Cancers (Basel). 2023. PMID: 37958430 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

