Optimizing Recombinant Protein Production in the Escherichia coli Periplasm Alleviates Stress
- PMID: 29654183
- PMCID: PMC5981079
- DOI: 10.1128/AEM.00270-18
Optimizing Recombinant Protein Production in the Escherichia coli Periplasm Alleviates Stress
Abstract
In Escherichia coli, many recombinant proteins are produced in the periplasm. To direct these proteins to this compartment, they are equipped with an N-terminal signal sequence so that they can traverse the cytoplasmic membrane via the protein-conducting Sec translocon. Recently, using the single-chain variable antibody fragment BL1, we have shown that harmonizing the target gene expression intensity with the Sec translocon capacity can be used to improve the production yields of a recombinant protein in the periplasm. Here, we have studied the consequences of improving the production of BL1 in the periplasm by using a proteomics approach. When the target gene expression intensity is not harmonized with the Sec translocon capacity, the impaired translocation of secretory proteins, protein misfolding/aggregation in the cytoplasm, and an inefficient energy metabolism result in poor growth and low protein production yields. The harmonization of the target gene expression intensity with the Sec translocon capacity results in normal growth, enhanced protein production yields, and, surprisingly, a composition of the proteome that is-besides the produced target-the same as that of cells with an empty expression vector. Thus, the single-chain variable antibody fragment BL1 can be efficiently produced in the periplasm without causing any notable detrimental effects to the production host. Finally, we show that under the optimized conditions, a small fraction of the target protein is released into the extracellular milieu via outer membrane vesicles. We envisage that our observations can be used to design strategies to further improve the production of secretory recombinant proteins in E. coliIMPORTANCE The bacterium Escherichia coli is widely used to produce recombinant proteins. Usually, trial-and-error-based screening approaches are used to identify conditions that lead to high recombinant protein production yields. Here, for the production of an antibody fragment in the periplasm of E. coli, we show that an optimization of its production is accompanied by the alleviation of stress. This indicates that the monitoring of stress responses could be used to facilitate enhanced recombinant protein production yields.
Keywords: Escherichia coli; Sec translocon; periplasm; proteomics; recombinant protein production.
Copyright © 2018 American Society for Microbiology.
Figures


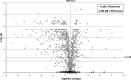
 , proteins in cells producing the scFv BL1 under optimized conditions;
, proteins in cells producing the scFv BL1 under optimized conditions;  , proteins in cells producing the scFv BL1 under nonoptimized conditions.
, proteins in cells producing the scFv BL1 under nonoptimized conditions.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

