Clinical use of lentiviral vectors
- PMID: 29654266
- PMCID: PMC6035154
- DOI: 10.1038/s41375-018-0106-0
Clinical use of lentiviral vectors
Abstract
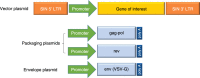
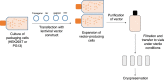
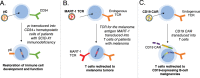
Viral vectors provide an efficient means for modification of eukaryotic cells, and their use is now commonplace in academic laboratories and industry for both research and clinical gene therapy applications. Lentiviral vectors, derived from the human immunodeficiency virus, have been extensively investigated and optimized over the past two decades. Third-generation, self-inactivating lentiviral vectors have recently been used in multiple clinical trials to introduce genes into hematopoietic stem cells to correct primary immunodeficiencies and hemoglobinopathies. These vectors have also been used to introduce genes into mature T cells to generate immunity to cancer through the delivery of chimeric antigen receptors (CARs) or cloned T-cell receptors. CAR T-cell therapies engineered using lentiviral vectors have demonstrated noteworthy clinical success in patients with B-cell malignancies leading to regulatory approval of the first genetically engineered cellular therapy using lentiviral vectors. In this review, we discuss several aspects of lentiviral vectors that will be of interest to clinicians, including an overview of lentiviral vector development, the current uses of viral vectors as therapy for primary immunodeficiencies and cancers, large-scale manufacturing of lentiviral vectors, and long-term follow-up of patients treated with gene therapy products.
Conflict of interest statement
MCM is an inventor on issued and pending patents related to T-cell immunotherapy utilizing lentiviral vectors for which he has received royalty payments. UOD declares that she has no conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

