On the use of Parylene C polymer as substrate for peripheral nerve electrodes
- PMID: 29654317
- PMCID: PMC5899141
- DOI: 10.1038/s41598-018-24502-z
On the use of Parylene C polymer as substrate for peripheral nerve electrodes
Abstract
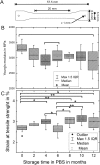
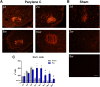
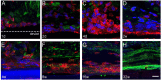
Parylene C is a highly flexible polymer used in several biomedical implants. Since previous studies have reported valuable biocompatible and manufacturing characteristics for brain and intraneural implants, we tested its suitability as a substrate for peripheral nerve electrodes. We evaluated 1-year-aged in vitro samples, where no chemical differences were observed and only a slight deviation on Young's modulus was found. The foreign body reaction (FBR) to longitudinal Parylene C devices implanted in the rat sciatic nerve for 8 months was characterized. After 2 weeks, a capsule was formed around the device, which continued increasing up to 16 and 32 weeks. Histological analyses revealed two cell types implicated in the FBR: macrophages, in contact with the device, and fibroblasts, localized in the outermost zone after 8 weeks. Molecular analysis of implanted nerves comparing Parylene C and polyimide devices revealed a peak of inflammatory cytokines after 1 day of implant, returning to low levels thereafter. Only an increase of CCL2 and CCL3 was found at chronic time-points for both materials. Although no molecular differences in the FBR to both polymers were found, the thick tissue capsule formed around Parylene C puts some concern on its use as a scaffold for intraneural electrodes.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Tyler, D. J., Polasek, K. H. & Schiefer, M. A. Peripheral Nerve Interfaces. Nerves and Nerve Injuries2 (Elsevier Ltd., 2015).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

