Measurement of cations, anions, and acetate in serum, urine, cerebrospinal fluid, and tissue by ion chromatography
- PMID: 29654634
- PMCID: PMC5899179
- DOI: 10.14814/phy2.13666
Measurement of cations, anions, and acetate in serum, urine, cerebrospinal fluid, and tissue by ion chromatography
Abstract
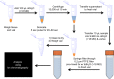
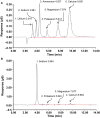
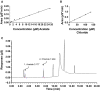
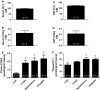
Accurate quantification of cations and anions remains a major diagnostic tool in understanding diseased states. The current technologies used for these analyses are either unable to quantify all ions due to sample size/volume, instrument setup/method, or are only able to measure ion concentrations from one physiological sample (liquid or solid). Herein, we adapted a common analytical chemistry technique, ion chromatography and applied it to measure the concentration of cations; sodium, potassium, calcium, and magnesium (Na+ , K+ , Ca2+ , and Mg2+ ) and anions; chloride, and acetate (Cl- , - OAc) from physiological samples. Specifically, cations and anions were measured in liquid samples: serum, urine, and cerebrospinal fluid, as well as tissue samples: liver, cortex, hypothalamus, and amygdala. Serum concentrations of Na+ , K+ , Ca2+ , Mg2+ , Cl- , and - OAc (mmol/L): 138.8 ± 4.56, 4.05 ± 0.21, 4.07 ± 0.26, 0.98 ± 0.05, 97.7 ± 3.42, and 0.23 ± 0.04, respectively. Cerebrospinal fluid concentrations of Na+ , K+ , Ca2+ , Mg2+ , Cl- , and - OAc (mmol/L): 145.1 ± 2.81, 2.41 ± 0.26, 2.18 ± 0.38, 1.04 ± 0.11, 120.2 ± 3.75, 0.21 ± 0.05, respectively. Tissue Na+ , K+ , Ca2+ , Mg2+ , Cl- , and - OAc were also measured. Validation of the ion chromatography method was established by comparing chloride concentration between ion chromatography with a known method using an ion selective chloride electrode. These results indicate that ion chromatography is a suitable method for the measurement of cations and anions, including acetate from various physiological samples.
Keywords: Anion; cations; electrolyte; ion chromatography.
© 2018 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures



References
-
- Avery, D. H. , Overall J. E., Calil H. M., and Hollister L. E.. 1983. Plasma calcium and phosphate during alcohol intoxication. Alcoholics versus nonalcoholics. J. Stud. Alcohol 44:205–214. - PubMed
-
- Christensen, J. D. , Barrere B. J., Boada F. E., Vevea J. M., and Thulborn K. R.. 1996. Quantitative tissue sodium concentration mapping of normal rat brain. Magn. Reson. Med. 36:83–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

