Advances in Evaluation of Chronic Diarrhea in Infants
- PMID: 29654747
- PMCID: PMC6044208
- DOI: 10.1053/j.gastro.2018.03.067
Advances in Evaluation of Chronic Diarrhea in Infants
Abstract
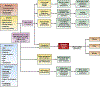
Diarrhea is common in infants (children less than 2 years of age), usually acute, and, if chronic, commonly caused by allergies and occasionally by infectious agents. Congenital diarrheas and enteropathies (CODEs) are rare causes of devastating chronic diarrhea in infants. Evaluation of CODEs is a lengthy process and infrequently leads to a clear diagnosis. However, genomic analyses and the development of model systems have increased our understanding of CODE pathogenesis. With these advances, a new diagnostic approach is needed. We propose a revised approach to determine causes of diarrhea in infants, including CODEs, based on stool analysis, histologic features, responses to dietary modifications, and genetic tests. After exclusion of common causes of diarrhea in infants, the evaluation proceeds through analyses of stool characteristics (watery, fatty, or bloody) and histologic features, such as the villus to crypt ratio in intestinal biopsies. Infants with CODEs resulting from defects in digestion, absorption, transport of nutrients and electrolytes, or enteroendocrine cell development or function have normal villi to crypt ratios; defects in enterocyte structure or immune-mediated conditions result in an abnormal villus to crypt ratios and morphology. Whole-exome and genome sequencing in the early stages of evaluation can reduce the time required for a definitive diagnosis of CODEs, or lead to identification of new variants associated with these enteropathies. The functional effects of gene mutations can be analyzed in model systems such as enteroids or induced pluripotent stem cells and are facilitated by recent advances in gene editing procedures. Characterization and investigation of new CODE disorders will improve management of patients and advance our understanding of epithelial cells and other cells in the intestinal mucosa.
Keywords: Detection; Gastrointestinal Disorder; Inherited; Pediatric.
Copyright © 2018 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest
The authors disclose no conflicts.
Figures



References
-
- Duggan CP, Jaksic T. Pediatric intestinal failure. N Engl J Med 2017;377:666–675. - PubMed
-
- Avery GB, Villavicencio O, Lilly JR, et al. Intractable diarrhea in early infancy. Pediatrics 1968;41:712–722. - PubMed
-
- Iyngkaran N, Abdin Z, Davis K, et al. Acquired carbohydrate intolerance and cow milk protein-sensitive enteropathy in young infants. J Pediatr 1979;95:373–378. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

