Effects of upstream shear forces on priming of platelets for downstream adhesion and activation
- PMID: 29654993
- PMCID: PMC5985216
- DOI: 10.1016/j.actbio.2018.04.002
Effects of upstream shear forces on priming of platelets for downstream adhesion and activation
Abstract
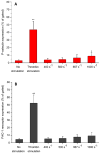
Platelets in flowing blood are sometimes exposed to elevated shear forces caused by anastomotic stenosis at the blood vessel-vascular implant interface. The objective of this study was to determine how effective upstream shear forces are in priming platelets for downstream adhesion and activation. Flow chambers with upstream stenotic regions (shear rates of 400-1000 s-1) were manufactured by relief molding of polydimethylsiloxane. Downstream from the stenotic regions, microcontact printing was used to covalently immobilize three different proteins (fibrinogen, collagen, or von Willebrand factor) to serve as platelet capture agents. Anticoagulated whole blood was perfused through the flow chambers and platelet adhesion to the downstream capture region was quantified. It was found that transient exposure of platelets to increased shear forces resulted in higher platelet adhesion on all three proteins. The duration of the platelet exposure to elevated shear forces was varied by changing the length of the stenotic regions. The results indicated that, in addition to the magnitude of shear forces, the duration of exposure to these forces was also an important factor in priming platelets. The effect of upstream shear forces on platelet activation was assessed by quantifying P-selectin, integrin αIIbβ3, lysosomal glycoprotein, and phosphatidylserine exposure using flow cytometry. The results suggested that increased shear forces were capable of increasing the priming of platelets for downstream activation. This study implicates the anastomotic region(s) of vascular implants as a locus of platelet pre-activation that may lead to thrombus formation downstream.
Statement of significance: A synthetic small-diameter vascular graft can often become stenotic due to intimal fibrous hyperplasia, either generally along the inside of the graft or at the anastomotic regions, leading to an increased shear force on flowing platelets. Our lab is studying how the upstream platelet preactivation (aka "priming") in flowing blood affects their downstream adhesion and activation. This manuscript describes a study in which priming of platelets is achieved by upstream stenotic narrowing in a microfluidic flow chamber. Such experimental design was intended to mimic a vascular implant with stenotic upstream anastomosis and downstream exposed platelet protein agonists. Understanding how the pre-activated platelets respond to imperfect vascular implant surfaces downstream is an important factor in designing better vascular implants.
Keywords: Anastomotic stenosis; Flow cytometry; Microcontact printing; Microfluidics; Platelet adhesion and activation.
Copyright © 2018 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Bertrand ME, Legrand V, Boland J, Fleck E, Bonnier J, Emmanuelson H, Vrolix M, Missault L, Chierchia S, Casaccia M, Niccoli L, Oto A, White C, Webb-Peploe M, McFadden EP. Randomized multicenter comparison of conventional anticoagulation versus antiplatelet therapy in unplanned and elective coronary stenting. Circulation. 1998;98:1597–1603. - PubMed
-
- Jackson MR, Johnson WC, Williford WO, Valentine RJ, Clagett GP. The effect of anticoagulation therapy and graft selection on the ischemic consequences of femoropopliteal bypass graft occlusion: results from a multicenter randomized clinical trial. J Vasc Surg. 2002;35:292–298. - PubMed
-
- Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand Factor. Cell. 1996;84:289–297. - PubMed
-
- Moroi M, Jung SM, Shinmyozu K, Tomiyama Y, Ordinas A, Diaz-Ricart M. Analysis of platelet adhesion to a collagen-coated surface under flow conditions: the involvement of glycoprotein VI in the platelet adhesion. Blood. 1996;88:2081–2092. - PubMed
-
- Godo MN, Sefton MV. Characterization of transient platelet contacts on a polyvinyl alcohol hydrogel by video microscopy. Biomaterials. 1999;20:1117–1126. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

