Measuring Clinical Treatment Response in Myasthenia Gravis
- PMID: 29655453
- PMCID: PMC6697147
- DOI: 10.1016/j.ncl.2018.01.006
Measuring Clinical Treatment Response in Myasthenia Gravis
Abstract
In this article we provide an overview of health-related outcome measurement-to better understand what different outcomes used in myasthenia actually measure-and to provide some guidance when choosing measures based on the clinical context and question. In myasthenia, the most commonly used outcome measures are aimed at assessing the signs and symptoms. In this review, we provide a summary of the most commonly used outcome measures. We discuss instruments that gauge disease overall health impact, such as on disability and quality of life. Finally, we discuss other relevant outcomes such as steroid-sparing effects and the role of surrogate markers.
Keywords: Disability; Minimal important difference; Myasthenia gravis; Outcome measurement; Responsiveness.
Copyright © 2018 Elsevier Inc. All rights reserved.
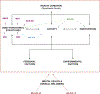
Figures
References
-
- Jette AM. Toward a common language for function, disability, and health. Phys Ther 2006;86(5):726–34. - PubMed
-
- World Health Organization. International classification of functioning, disability and health (ICF) 1st edition. Geneva: World Health Organization; 2002. Available at: http://www.who.int/classifications/icf/icfbeginnersguide.pdf?ua=1. Accessed June, 2017.
-
- Kirshner B, Guyatt G. A methodological framework for assessing health indices. J Chronic Dis 1985;38(1):27–36. - PubMed
-
- King MT. A point of minimal important difference (MID): a critique of terminology and methods. Expert Rev Pharmacoecon Outcomes Res 2011;11(2):171–84. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

