Endocrine and haemodynamic changes in resistant hypertension, and blood pressure responses to spironolactone or amiloride: the PATHWAY-2 mechanisms substudies
- PMID: 29655877
- PMCID: PMC5966620
- DOI: 10.1016/S2213-8587(18)30071-8
Endocrine and haemodynamic changes in resistant hypertension, and blood pressure responses to spironolactone or amiloride: the PATHWAY-2 mechanisms substudies
Erratum in
-
Correction to Lancet Diabetes Endocrinol 2018; 6: 464-75.Lancet Diabetes Endocrinol. 2018 Aug;6(8):e16. doi: 10.1016/S2213-8587(18)30211-0. Lancet Diabetes Endocrinol. 2018. PMID: 30053988 Free PMC article. No abstract available.
Abstract
Background: In the PATHWAY-2 study of resistant hypertension, spironolactone reduced blood pressure substantially more than conventional antihypertensive drugs. We did three substudies to assess the mechanisms underlying this superiority and the pathogenesis of resistant hypertension.
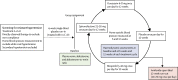
Methods: PATHWAY-2 was a randomised, double-blind crossover trial done at 14 UK primary and secondary care sites in 314 patients with resistant hypertension. Patients were given 12 weeks of once daily treatment with each of placebo, spironolactone 25-50 mg, bisoprolol 5-10 mg, and doxazosin 4-8 mg and the change in home systolic blood pressure was assessed as the primary outcome. In our three substudies, we assessed plasma aldosterone, renin, and aldosterone-to-renin ratio (ARR) as predictors of home systolic blood pressure, and estimated prevalence of primary aldosteronism (substudy 1); assessed the effects of each drug in terms of thoracic fluid index, cardiac index, stroke index, and systemic vascular resistance at seven sites with haemodynamic monitoring facilities (substudy 2); and assessed the effect of amiloride 10-20 mg once daily on clinic systolic blood pressure during an optional 6-12 week open-label runout phase (substudy 3). The PATHWAY-2 trial is registered with EudraCT, number 2008-007149-30, and ClinicalTrials.gov, number NCT02369081.
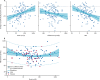
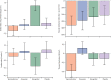
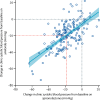
Findings: Of the 314 patients in PATHWAY-2, 269 participated in one or more of the three substudies: 126 in substudy 1, 226 in substudy 2, and 146 in substudy 3. Home systolic blood pressure reduction by spironolactone was predicted by ARR (r2=0·13, p<0·0001) and plasma renin (r2=0·11, p=0·00024). 42 patients had low renin concentrations (predefined as the lowest tertile of plasma renin), of which 31 had a plasma aldosterone concentration greater than the mean value for all 126 patients (250 pmol/L). Thus, 31 (25% [95% CI 17-33]) of 126 patients were deemed to have inappropriately high aldosterone concentrations. Thoracic fluid content was reduced by 6·8% from baseline (95% CI 4·0 to 8·8; p<0·0001) with spironolactone, but not other treatments. Amiloride (10 mg once daily) reduced clinic systolic blood pressure by 20·4 mm Hg (95% CI 18·3-22·5), compared with a reduction of 18·3 mm Hg (16·2-20·5) with spironolactone (25 mg once daily). No serious adverse events were recorded, and adverse symptoms were not systematically recorded after the end of the double-blind treatment. Mean plasma potassium concentrations increased from 4·02 mmol/L (95% CI 3·95-4·08) on placebo to 4·50 (4·44-4·57) on amiloride (p<0·0001).
Interpretation: Our results suggest that resistant hypertension is commonly a salt-retaining state, most likely due to inappropriate aldosterone secretion. Mineralocorticoid receptor blockade by spironolactone overcomes the salt retention and resistance of hypertension to treatment. Amiloride seems to be as effective an antihypertensive as spironolactone, offering a substitute treatment for resistant hypertension.
Funding: British Heart Foundation and UK National Institute for Health Research.
Copyright © 2018 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures


Comment in
-
Fluid retention, aldosterone excess, and treatment of resistant hypertension.Lancet Diabetes Endocrinol. 2018 Jun;6(6):431-433. doi: 10.1016/S2213-8587(18)30080-9. Epub 2018 Apr 11. Lancet Diabetes Endocrinol. 2018. PMID: 29655878 No abstract available.
-
Investigation of primary aldosteronism in patients with resistant hypertension.Lancet Diabetes Endocrinol. 2018 Aug;6(8):599-600. doi: 10.1016/S2213-8587(18)30173-6. Lancet Diabetes Endocrinol. 2018. PMID: 30053983 No abstract available.
-
Investigation of primary aldosteronism in patients with resistant hypertension - Authors' reply.Lancet Diabetes Endocrinol. 2018 Aug;6(8):600-601. doi: 10.1016/S2213-8587(18)30174-8. Lancet Diabetes Endocrinol. 2018. PMID: 30053984 No abstract available.
References
-
- Myat A, Redwood SR, Qureshi AC, Spertus JA, Williams B. Resistant hypertension. BMJ. 2012;345:e7473. - PubMed
-
- Achelrod D, Wenzel U, Frey S. Systematic review and meta-analysis of the prevalence of resistant hypertension in treated hypertensive populations. Am J Hypertens. 2015;28:355–361. - PubMed
-
- Dickerson JEC, Hingorani AD, Ashby MJ, Palmer CR, Brown MJ. Optimisation of antihypertensive treatment by crossover rotation of four major classes. Lancet. 1999;353:2008–2013. - PubMed
-
- Dahal K, Kunwar S, Rijal J. The effects of aldosterone antagonists in patients with resistant hypertension: a meta-analysis of randomized and nonrandomized studies. Am J Hypertens. 2015;28:1376–1385. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

