Monocyte function in patients with myelodysplastic syndrome
- PMID: 29656609
- PMCID: PMC6113098
- DOI: 10.1002/JLB.5AB1017-419RR
Monocyte function in patients with myelodysplastic syndrome
Abstract
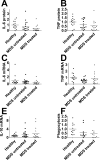
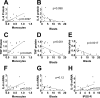
Myelodysplastic syndrome (MDS) is a malignant hematopoietic stem cell disorder that frequently evolves into acute myeloid leukemia (AML). Patients with MDS are prone to infectious complications, in part due to the presence of severe neutropenia and/or neutrophil dysfunction. However, not all patients with neutropenia become infected, suggesting that other immune cells may compensate in these patients. Monocytes are also integral to immunologic defense; however, much less is known about monocyte function in patients with MDS. In the current study, we monitor the composition of peripheral blood monocytes and several aspects of monocyte function in MDS patients, including HLA-DR expression, LPS-induced inflammatory cytokine production, and phagocytosis. We find that monocytes from MDS patients exhibit relatively normal innate immune functions compared to monocytes from healthy control subjects. We also find that HLA-DR expression is moderately increased in monocytes from MDS patients. These results suggest that monocytes could compensate for other immune deficits in MDS patients to help fight infection. We also find that the range of immune functions in monocytes from MDS patients correlates with several key clinical parameters, including blast cell count, monocyte count, and revised International Prognostic Scoring System score, suggesting that disease severity impacts monocyte function in MDS patients.
Keywords: LPS; PBMC; human leukocyte antigen-antigen D related; myelodysplastic syndrome; phagocytosis.
©2018 Society for Leukocyte Biology.
Conflict of interest statement
Figures

References
-
- Tefferi A, Vardiman JW. Myelodysplastic syndromes. The New England journal of medicine. 2009;361:1872–85. - PubMed
-
- Barzi A, Sekeres MA. Myelodysplastic syndromes: a practical approach to diagnosis and treatment. Cleve Clin J Med. 2010;77:37–44. - PubMed
-
- Garcia-Manero G. Myelodysplastic syndromes: 2011 update on diagnosis, risk-stratification, and management. American journal of hematology. 2011;86:490–8. - PubMed
-
- Corey SJ, Minden MD, Barber DL, Kantarjian H, Wang JC, Schimmer AD. Myelodysplastic syndromes: the complexity of stem-cell diseases. Nat Rev Cancer. 2007;7:118–29. - PubMed
-
- Sekeres MA. The epidemiology of myelodysplastic syndromes. Hematology/oncology clinics of North America. 2010;24:287–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

