Synaptotagmin 4 Regulates Pancreatic β Cell Maturation by Modulating the Ca2+ Sensitivity of Insulin Secretion Vesicles
- PMID: 29656931
- PMCID: PMC5962294
- DOI: 10.1016/j.devcel.2018.03.013
Synaptotagmin 4 Regulates Pancreatic β Cell Maturation by Modulating the Ca2+ Sensitivity of Insulin Secretion Vesicles
Abstract
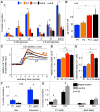
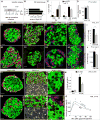
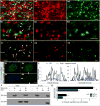
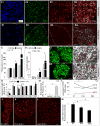
Islet β cells from newborn mammals exhibit high basal insulin secretion and poor glucose-stimulated insulin secretion (GSIS). Here we show that β cells of newborns secrete more insulin than adults in response to similar intracellular Ca2+ concentrations, suggesting differences in the Ca2+ sensitivity of insulin secretion. Synaptotagmin 4 (Syt4), a non-Ca2+ binding paralog of the β cell Ca2+ sensor Syt7, increased by ∼8-fold during β cell maturation. Syt4 ablation increased basal insulin secretion and compromised GSIS. Precocious Syt4 expression repressed basal insulin secretion but also impaired islet morphogenesis and GSIS. Syt4 was localized on insulin granules and Syt4 levels inversely related to the number of readily releasable vesicles. Thus, transcriptional regulation of Syt4 affects insulin secretion; Syt4 expression is regulated in part by Myt transcription factors, which repress Syt4 transcription. Finally, human SYT4 regulated GSIS in EndoC-βH1 cells, a human β cell line. These findings reveal the role that altered Ca2+ sensing plays in regulating β cell maturation.
Keywords: Ca(2+); Myt1; Syt4; diabetes; docking; insulin; maturation; membrane fusion; secretion; vesicle.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Synaptotagmins Tweak Functional β Cell Maturation.Dev Cell. 2018 May 7;45(3):284-286. doi: 10.1016/j.devcel.2018.04.018. Dev Cell. 2018. PMID: 29738707 Free PMC article.
References
-
- Aguayo-Mazzucato C, Zavacki AM, Marinelarena A, Hollister-Lock J, El Khattabi I, Marsili A, Weir GC, Sharma A, Larsen PR, Bonner-Weir S. Thyroid hormone promotes postnatal rat pancreatic beta-cell development and glucose-responsive insulin secretion through MAFA. Diabetes. 2013;62:1569–1580. - PMC - PubMed
-
- Ammala C, Ashcroft FM, Rorsman P. Calcium-independent potentiation of insulin release by cyclic AMP in single beta-cells. Nature. 1993;363:356–358. - PubMed
-
- Andersson SA, Olsson AH, Esguerra JL, Heimann E, Ladenvall C, Edlund A, Salehi A, Taneera J, Degerman E, Groop L, et al. Reduced insulin secretion correlates with decreased expression of exocytotic genes in pancreatic islets from patients with type 2 diabetes. Molecular and cellular endocrinology. 2012;364:36–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK097392/DK/NIDDK NIH HHS/United States
- R01 DK090570/DK/NIDDK NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- R01 DK106228/DK/NIDDK NIH HHS/United States
- R01 DK065949/DK/NIDDK NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- R01 DK105831/DK/NIDDK NIH HHS/United States
- R01 DK108666/DK/NIDDK NIH HHS/United States
- R01 DK050203/DK/NIDDK NIH HHS/United States
- P30 DK019525/DK/NIDDK NIH HHS/United States
- R01 DK115620/DK/NIDDK NIH HHS/United States
- U01 DK089523/DK/NIDDK NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- R01 DK125696/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

