The Platelet Lifeline to Cancer: Challenges and Opportunities
- PMID: 29657130
- PMCID: PMC5997503
- DOI: 10.1016/j.ccell.2018.03.002
The Platelet Lifeline to Cancer: Challenges and Opportunities
Abstract
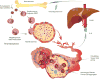
Besides their function in limiting blood loss and promoting wound healing, experimental evidence has highlighted platelets as active players in all steps of tumorigenesis including tumor growth, tumor cell extravasation, and metastasis. Additionally, thrombocytosis in cancer patients is associated with adverse patient survival. Due to the secretion of large amounts of microparticles and exosomes, platelets are well positioned to coordinate both local and distant tumor-host crosstalk. Here, we present a review of recent discoveries in the field of platelet biology and the role of platelets in cancer progression as well as challenges in targeting platelets for cancer treatment.
Keywords: anti-platelet therapy; aspirin; biomarker; cancer; cancer therapy; metastasis; microparticles; platelets; thrombocytosis; tumor angiogenesis.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures



References
-
- Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. The Lancet Oncology. 2012;13:518–527. - PubMed
-
- Amisten S. A rapid and efficient platelet purification protocol for platelet gene expression studies. Methods in molecular biology. 2012;788:155–172. - PubMed
-
- Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Developmental cell. 2011;21:193–215. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

